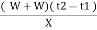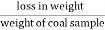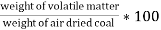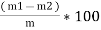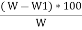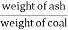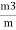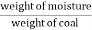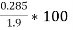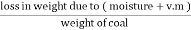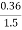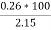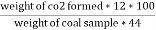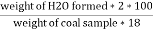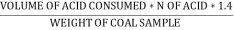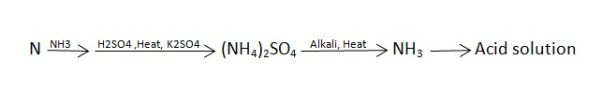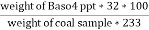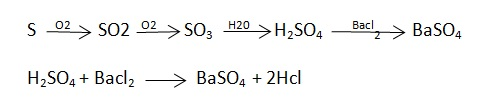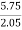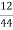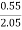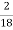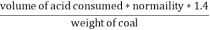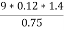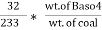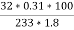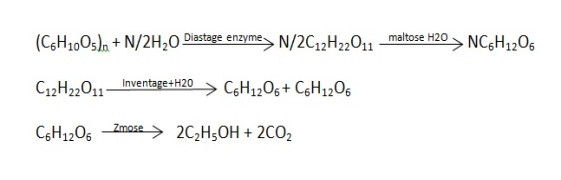UNIT 4
FULES AND COMBUSTION
Definition :-
Fuels :-
A fuel is the substance which on combustion produces a large amount of heart.
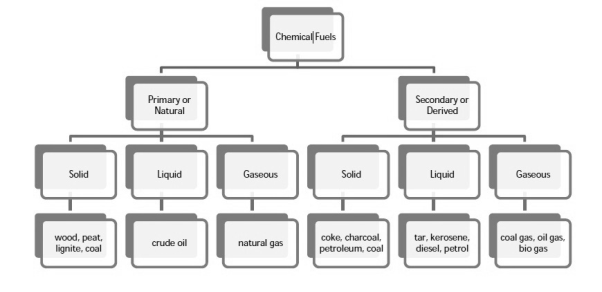
Classification of chemical fuels :-
Chemical fuels are classified on the basis of occurrence into :-
- Primary ( natural )
- Secondary ( derived ) types / manmade .
The secondary fuels are obtained from primary fuel by processing or they are manmade. e.g.:- charcoal is obtained from wood by partial combustion of wood , ethyl alcohol is obtained by fermentation of carbohydrates .
Both the primary and secondary fuels are further classified on the basis of physical state into solid liquid and gaseous fuels.
|
Extra definitions :-
1] fuels :-
Fuels are defined as materials that create usable energy through chemical nuclear or electrochemical reaction.
2] chemical fuel :-
The fossil fuels , wood vegetal oils etc. . which produce heat on burning are known as chemical fuels.
Comparison between solid , liquid , and gaseous fuels.
SOLID FUELS | LIQUID FUELS | GASEOUS FUELS |
|
|
|
|
|
|
Calorific value and its units
Calorific value :- efficiency of combustible fuels can be measured in terms of their calorific value . it is the heat evolved on combustion of a unit quantity of fuel.
Definition of calorific value :-
- It is defined as the amount of heat obtained on complete combustion of unit mass of a solid or liquid fuel or unit volume of a gaseous fuel at STP.
Gross calorific value ( G.V.C ) :-
- Usually fuel contains some hydrogen . the hydrogen atoms are bonded to carbon atoms in the fuel when the fuel is burnt , hydrogen forms water vapors . the water vapors if cooled , we get certain amount of heat as the water has high latent heat of 2450 joules / gm or 587 Cal / gm.
- Thus, during the study of calorific value of a fuel we get some heat directly by combustion of fuel and in addition we get certain amount of heat by cooling the products of combustion to 15-degree c.
Gross calorific value :-
- Gross calorifia value of a fuel can be defined as the total amount of heat obtained on complete combustion of unit mass of a solid or liquid fuel or unit volume of a gaseous fuel ( S T P ) and on cooling the products of combustion to 15 degree c . the gross calorific value is also called as higher calorific value.
- The G.V.C is of only theoretical importance because in actual practice we do not have any provision of cooling the products of combustion during combustion of fuel in an engine , furnace or any other fuel burning device.
Net calorific value :-
- A fuel contain hydrogen produces water heat and actually less heat is available for heating.
- There is not any furnace , engine or device designed to collect the heat being taken away by the water vapors . therefore, practically we get lower calorific value than the theoretical expected.
Net calorific value :-
- Net calorific value is defined as the amount of heat obtained practically on complete combustion of unit mass of solid or liquid fuel or unit mass of solid or liquid fuel or unit volume of a gaseous fuel at step and the products of combustion are allowed to escape with some heat N.C.V is also called as lower calorific value.
The N.C.V and G.C.V are related as under
G.C.V = N.C.V + 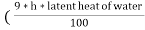 )
)
Where h is the percentage of hydrogen in the fuel.
It should be noted that the unit of latent heat of water and unit of G.V.C , N.C.V should be same .
Units of calorific value :-
system | Solid or liquid fuel | Gaseous fuel |
CGS | Cal / gm | Cal / liter |
MKS | Kcal / kg | Kcal / |
S.I | Joules / kg | Joules / |
Determination of calorific value :-
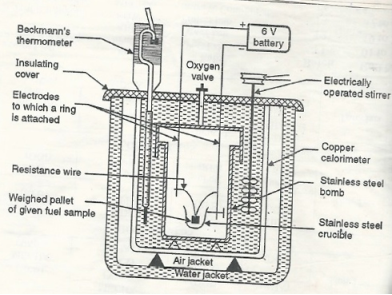
The gross calorific value of solid fuels and liquid fuels can be determined by bomb calorimeter .( if the liquid is volatile , then it is filled in a polythene capsule of negligible mass then used in experiment.
Construction :- a bomb colorimeter consists of
- Bomb pot
- Calorie meter
- Water and air jackets
- Accessories
- Pellet press
- Oxygen cylinder
Bomb pot :-
It is a cylindrical strong stainless-steel pot having a lid . the lid can be fitted air. Tight to bomb pot by screwing.
- There are two type of electrodes fitted through lid and there is an oxygen inlet valve as its center.
- One of these electrodes is provided with a ring to hold the crucible containing fuel .there is thin resistance wire tied to the electrodes in loop form and the loop touches the fuel.
- The weighed fuel is burnt in the bomb pot in the presence of high-pressure oxygen.
Calorimeter :-
- There is a stainless steel or copper calorimeter in which the bomb pot is kept .it contains a known volume of water and the water is kept circulating around the bomb pot with the help of a stirrer.
- A Beckman thermometer or digital thermometer is kept in the water of calorimeter , which can record the rise in temperature of welter due to absorb in a heat generated .
- There are insulator stands between calorimeter and water jacket.
Accessories :-
- There is a pellet press to convert the powder of solid fuel to pellet form .for a liquid fuel a capsule of negligible weight can be used.
- There is an oxygen cylinder with pressure gauge to fill oxygen in the bomb pot at the pressure of nearly 25 kg / cm².
- There is also a D.C battery of a about 6 volts to start combustion of fuel.
|
Fig: Bomb Caloriemeter
Working :-
- Weigh the pellet of solid fuel or liquid capsule and keep it in the crucible .keep the crucible in the ring of the electrode . keep the resistance wire touching to the fuel.
- Add about 10 ml of distilled water at the bottom of bomb pot and fix the lid tightly to bomb by screwing.
- Fill the bomb with oxygen at the pressure about 25 kg / cm².
- Place the bomb in calorimeter add known volume of water in the calorimeter so that the bomb gets immersed in the water.
- Place the calorimeter in the water jacket over the plastic studs .keep the thermometer and stirrer in the water of calorimeter.
- Put the plastic cover on the and make electrical connections from battery to electrodes.
- Operate the stirrer for s minutes and note the initial temperature of water ( t1° c ).
- Pass the current for about 5 – 10 seconds to heat the wire so that the fuel catches fire. If the fuel contains S and N elements, they get converted to SO3 and N2O5 .these gases get dissolved in the distilled water in bomb to form H2SO4 and HNO3 ( along with liberation little heat ).
- Note the maximum temperature reached .after that note the rate of fall of temperature per minute and the time taken for reaching to initial temp. are noted.
- Open the bomb pot and wash the contents at its bottom into a beaker to find out the amount of H2SO4 and HNO3 formed.
Calculations :-
Gross calorific value of fuel = l calories / gm
Rise in temperature of water = ( t2 – t1 )
Heat liberated by burning fuel = heat absorbed by water and calorimeter
Therefore , XL = ( W+W) ( T2 – T1 ) G.CV = L =
|
Note :-
Water equivalent of calorimeter – set ( w ) is determined by burning fuel of known gross calorific value and using the above equation.
Standard gross calorific values of some pure fuels are,
Naphthalene = 9622 Cal / gm
Benzoic acid = 6325 Cal / gm
Camphor = 9292 Cal / gm
Salicylic acid = 5269 Cal /gm
NCV for the fuel is calculated as below .
If H is percentage of hydrogen in the fuel, then the heat taken by water formed during combustion to convert it into steam is = 0.09h * 587 Cal / gm
And
NCV = GCV – 0.09h * 587 Cal / gm or kcal / kg.
Corrections :-
To get more accurate results , the following corrections should be considered.
- Fuse wire correction ( F )
- Acid correction ( a )
- Cooling correction ( tc )
Out of the total obtained little heat is given out by fuse wire when the current is passed for s – 10 sec .to start the combustion. Hence it must be subtracted. Are exothermic and the heat measured includes a small share by these acid formation.
2. Cooling correction ( te ) :- If the time taken for water in calorimeter to cool from maximum temperature attained to the room. Temperature is t minutes and average cooling rate is dt / min , then the cooling correction to be added to rise in temperature is t.dt .
|
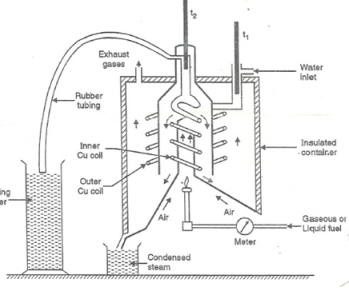
This calorimeter is used to measure calorific value of gaseous fuels and highly volatile fuels.
Principle :-
The gaseous fuel is burnt at a known constant rate in the calorimeter under such conditions that entire amount of heat produced is absorbed by water.
- Gas burner
- Combustion chamber ( chimney )
- Thermometers
- Insulating cover
- Gas burner :-
- There is a gas burner in which a known volume of gas is burnt at a known pressure .the gas is burnt at the rate of 3 – 4 lit. per minute.
3. Combustion chamber ( chimney ) :-
Around the burner there Is a combustion chamber which has a copper tubing coiled inside as well as outside of it water enters from top of the outer coils , moves to bottom of chimney and then goes up through the inner coil to the exit at top.
4. Thermometer :-
There are two thermometer to measure temperatures of inlet water and outlet water.
5. Insulating cover :-
The assembly is covered with by an insulator to detach combustion chamber from atmosphere .there are three holes for exhaust gas, water inlet and condensed steam.
Working :-
- Start burning the gas at suitable pressure and adjust the rate of water flow such that the temperature of outgoing water remains constant.
- Burn the gas for 5 – 10 minutes to have the steady temperatures in and around the combustion chamber.
- After the steady ( temperature in and around the combustion chamber ) conditions of outgoing.
A) Volume of gas burnt at given temperature and pressure in certain time period.
B) Quantity of water passed through coil during this period.
C) Mass of water condensed from product gas during the period.
D) The steady rise in temperature of water ( t2 – t1 )
|
Fig: Boy’s gas calorimeter
Calculations :-
- First convert the volume of gas burnt to volume of gas at STP .let this STP volume be V
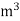 .
. - Let W = mass of cooling water used in the period of observation in Kg
- Let m= mass of water condensate in kg
- L= G.C.V OF THE FUEL
Heat produced by combustion of fuel = heat absorbed by cooling water
( assuming no heat loss in the steady state conditions )
Vl = w ( t2 – t1 )
L = 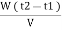 Kcal /
Kcal / 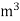
The mass of condensate water per m of gas will be m/v kg /m .
If this water had left as steam in product gases it would have taken away heat.
=  *587 kcal /
*587 kcal / 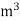
Therefore ,
NCV = GCV -  *587
*587
NCV = 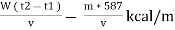
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
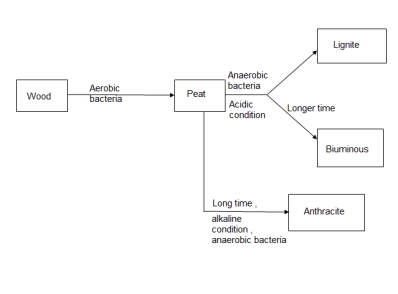
Solid fuels :- coal Coal is highly carbeanous matter formed vegetable matter buried in geomorphic changes , under pressure , by action of aerobic and anaerobic bacteria for long time.
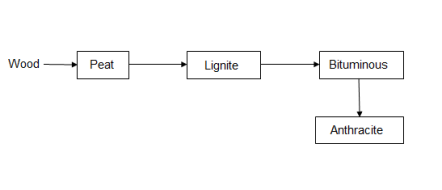
3. The processes of conversion of wood into coal is known as coalification.
4. In general coal contains mainly carbon atoms with mostly sp² hybridization. Atoms like O , H , S , N are covalently bonded to carbon atoms . coal also contain water and mineral particles entrapped.
5. When coal start burning , some of the heat produced is utilized to evaporate water and minerals are left behind . as ash ( metal oxidize , silica )
6. The volatile matter is the unburnt decomposed coal molecules fragments of colloidal size.
Classification of coal ( types / grades ):-
The conversion of wood ( plant matter ) into coal takes place progressively . depending upon the extent of transformation coals are divided into 4 types or grades or ranks. During the progressive conversion from peat to anthracite there is, Increase in carbon percentage calorific value , density luster , hardness , black color intensity decreases in – moisture , volatile , matter .
Peat :-
Uses :-
Lignite :-
Uses :-
Bituminous coal :-
2. Bituminous coal :-
3. Semi bituminous coal :-
( caking – coal decomposing at about 400°c melting forming a plastic mass through which U.M blows out forming highly swollen open structured coal called as coke )
Uses :- Making of coke , high temperature heating , coal gas for tar and chemicals.
4. Anthracite coal :-
Uses :-
Analysis of coal :- Coal is mainly composed of C , h , N , S moisture volatile matter. The purpose for coal analysis is
A sample of coal taken out from coal mine is analyzed in two ways.
Proximate analysis of coal ( 5 / 6 m ) Proximate analysis is the study or analysis of coal sample in which
A] moisture ( percent ) :-
Moisture percent =
B] volatile matter (V.M) :-
Volatile matter (percent ) :- =
The volatile matter percent can be also determined by taking the fresh weight of the air dried coal but the loss in weight .at 925°c will be due to loss of moisture and volatile matter if W is the mass of coal left at 925° c heating, Then , Volatile matter percent = =
C] Ash percent :-
Therefore , Ash ( percent
D] fixed carbon ( percent ) :- It is found by calculation f.c = 100 – ( moisture + v.m + ash )
significance ( important of proximate analysis )
Moisture :-
Volatile matter :-
However , the coals containing 15 – 25 percent of X.M on carbonization gives coke oven gas which is the source of various organic aromatic chemicals such coals have good caking property and caking the coals be obtained from the coals. Overall , regarding burning of coal the coal the coal with lesser V.M is better quality coal.
Ash :-
Principle of ash :-
D ] fixed carbon :-
f.c( percent ) = 100 – ( moisture + V.M + ash )
numerical :-
Given data :- M=1.9 gm ( m – m1 )= 0.285 gm ( moisture loss ) Loss in weight due to moisture and VM = 0.36gm
Weight of coal = 2.15 gm Weight of ash = 0.26gm
=
2. V.M ( percent ) :- v.m =
= = 9 percent
3. Ash ( percent ) :-
Therefore ,
Ash =
=
= 12 ( percent )
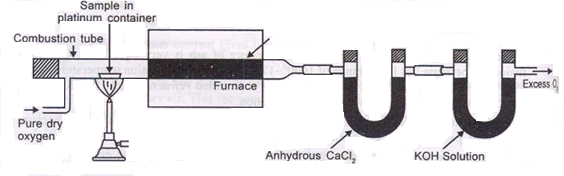
Principle :- The C in the coal gets converted co2 and hydrogen to h2o vapors burning in the presence of pure o2
Ultimate analysis of coal :-
The analysis of coal in which percentage of C , N , H , S and O elements are found out is known as ultimate analysis .
A] C,H in coal :-
C + O2 – CO 2 2H + ½ - H2O 2KoH + CO2 – K2CAO3 + H2O Cacl2 + 7H2O – Cacl2 – CacL2 .7h20
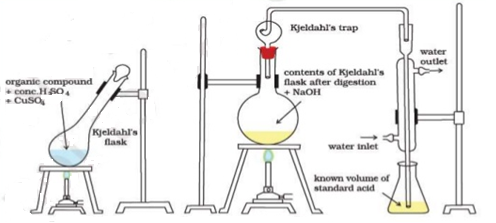
N ( percent ) – n in coal gets converted to anomnoussulphate by action of hot conc . H2SO4 and then on treatment with alkali solution .
C ( percent ) =
H ( percent ) =
B] nitrogen in coal :-
The unused acid is determined by titration with NaOH solution.
N ( percent ) =
C] Sulphur in coal :-
S ( percent ) =
Importance of ultimate analysis:-
Carbon :-
Hydrogen :-
Nitrogen :-
Sulphur :-
Oxygen :-
Numerical :-
Given data :- Increase in weight of any hydrous calcl2 = 0.55 Mass of coal = 0.75gms Volume of 0.12 NHCL consumed by NH3 = 50 – 41 = 9 ml
Mass of coal = 1.8gms Weight of Baso4 = 0.31 gm
Therefore , C percent ) =
Increase in weight of any hydrous Cacl2 = weight of H2O formed Mass of coal = 2.05gm H ( percent ) =
= 2.98 ( percent )
2. N ( percent ) = =
= 2.02 ( percent )
3. S ( percent ) =
=
= 2.36 ( percent )
|
Theoretical calorific values
A] gouthal formula :-
Theoretically calorific value of coal can be calculated from proximate analysis data by using gouthal formula .
G.C.V Cal / gm = 82fc + a.VM
The values of constant are as under
Petroleum :-
- Petroleum or crude oil is a dark greenish brown viscous liquid in earth crust at the depth of few meters to 7kms.
- Crude oil is the most important source of various liquid fuels used now and a large number of petrochemicals can be manufactured ( plastics , rubber , fibers , medicine ) from the crude oil fractions.
Origin of petroleum :-
- As per modern theory petroleum is formed from buried debris of plants and animals ( organic matter ) under favorable conditions.
- The burial of the organic matter during volcano upheavals. In earth surface should take place along with large quantity of water and under a dome of hard , impervious rock.
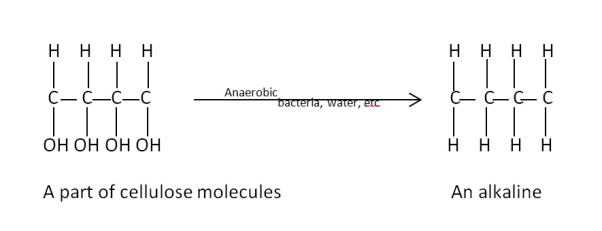
- An aerobic bacteria , higher temperature , radioactive matter makes the degradation of organic matter , in the presence of water , under the dome to highly alkanes rich matter as crude oil.
- The anaerobic bacteria take out oxygen atoms from cellulose protein oil molecules and forms alkenes rich crude oil
- E.g. –
|
- Prolonged action of higher temperature and action of radioactive substances cause the breaking very long hydrocarbon molecules to smaller during the ages of time , the hydrocarbon molecule.
- Crude oil is always deposited on the layer of salty water and above crude oil layer there is natural gas consisting of C1 TO C4 hydrocarbons and NH3 , H2S , CO , moisture.
Composition of petroleum :-
Average elemental composition of petroleum crude oil is,
- C = 80 -87 percent
- H= 11 to 15 percent
- O = 0.1 to 0.9 percent
- S = 0.1 to 3 percent
- N = 0.4 to 0.9 percent
Petroleum contains following type of compounds :-
1) Open chain alkanes
2) Cycloalkanes
3) Aromatics
4) Asphaltenes
5) Resins
Open chain alkenes :-
Both straight chain and branched alkanes are present in crude oil. In some crude oil such alkenes are major part.
Aromatics :-
- In all the crude oils benzene and alkyl substituted benzenes are up to 2 percent.
Asphaltenes :-
- All the crude oil contains small amount of poly condensed aromatic solid as colloidal dispersion in crude oil .the asphaltenes also contain N ,S , O atoms.
Resins :-
- These are polymeric substances .they are gummy and are lower molecular weight polymers . they are also containing N , S , O atoms .
- All the crude oil contain little amount of emulsified water is rich with dissolved salts.
- The number of carbon atoms in hydrocarbon molecules range from C1 TO C70 in crude oil.
Refining of petroleum :-
- Crude oil cannot be directly used for any purpose .refining or fractionation is the processes of separation of various fraction from crude oil , on the basis of fractional distillation .
- The crude oil contains a large number of hydrocarbon but by the processes of refining they are divided into few groups of mol.wt or boiling point ranges.
They are as follows :-
- Removal of water
- Removal of Sulphur
- Fractionation
|
Removal of water :-
First the emulsified water along with salts dissolved is removed by passing the crude oil between highly charged electrodes .The colloidal water droplets unite to form large drops which separate from oil.
Removal of Sulphur :-
Then the crude oil is treated with hot to remove .Sulphur from the Sulphur compound in crude oil.
Fractionation :-
The principle of fractional distillation is that the vapors of higher boiling point compounds get condensed into liquid , during the stepwise cooling.
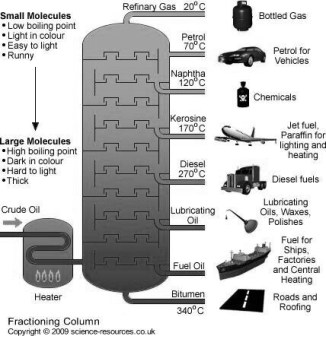
Construction :-
- The refining is carried out in a tall cylindrical tower called as fractionating column .the fractionating column is a tall cylindrical stainless-steel tower about 30 m in height and 3 m in diameter.
- There are 50 -60 horizontal stainless trays at about half meter distances in the tower. Each dry is provided with 4-5 bubble cups with loose caps so that the raising vapors through the cap bubble in the liquid deposited on tray.
- Temperature of the tower is 400° c near bottom, and it decrease gradually to 40°c atop .
The tower is cooled by open air.
Working :-
- The crude oil is heated at 400°c in a furnace or pipe still to convert it into vapors.
- The vapors travel upwards through the bubble caps and gradually get cooled the vapors of organic compounds with higher b.p get condensed in bubble caps and the liquid deposits on the trays.
- The uncondensed vapors rise up and get condensed turn by turn on upper bubble trays. There is vigorous bubbling action on each tray. In case large part of the vapors get condensed on a tray .then the extra liquid flow down to higher temp. lower trays and gets evaporated.
- From some bubble trays the fractions like petrol d, diesel , kerosene , naphtha , heavy oil etc. are taken out. Finally, a small of vapors comes out as uncondensed gases from the top where temp. is about 40 ° c.
- Heavy oil fraction can be further to get lubricating oils , Vaseline wax.
- The petrol obtained from refinery is called as straight run petrol but is not a good quality petrol.
- It is possible to convert any higher boiling fraction to convert into petrol or LPG by the processes know as cracking .
The various petroleum fraction has calorific value about 1100 Cal / gm .their composition, boiling range and uses are given.
Name of fraction | Boiling range | Composition of HC | uses |
| Below 40 ° c | C1 to c4 | Domestic and industrial fuel under lpg name |
2. Petroleum ether | 40-70° c | C5 to c7 | Fuel for Aeroplan helicopter as solvent |
3. Petrol or gasoline | 60-120° c | C5 to c8 | Fuel for petrol engines dry cleaning as solvent. |
4. Naphtha or solvent spirit | 120 -180 ° c | C7 to C10 | For dry cleaning for chemicals |
5. Kerosene | 180-250° c | C10 TO C16 | For domestic fuel for oil gas. |
6. Diesels | 250 - 320° c | C15 to c18 | Diesel engine fuel |
7. Heavy oil
| 320-400° c | C18 – c20
| Lubricating purpose |
8. residue |
| Above C30 | Road making water proofing |
Natural Gas is a fossil fuel. It occurs naturally and approximately consists of 95% hydrocarbon methane, the other 5% consists of nitrogen, carbon dioxide, helium or hydrogen sulfide. It takes millions of years for it to form.
The gas is formed when layers of decaying plants and animals are buried under the earth’s surface and are exposed to intense heat and pressure for millions of years. Plants originally obtain energy from the sun and store in the form of chemical bonds in the gas. And thus, the formation of this gas occurs.
Properties:
- The state of matter of this gas is gaseous.
- It doesn’t have any color and is a tasteless gas.
- It is free of any kind of toxic, there is no smoke on burning and it has high calorific value.
- The gas is odourless. However, a chemical called mercaptan is added to it in small amounts to give it distinctive smell of eggs. This helps to find out any gas leaks.
- It is a combustible gas and a fossil fuel.
- Its a mixture of simple hydrocarbon compounds.
- It contains primarily methane, along with small amounts of ethane, butane, pentane, and propane.
- The by-products of this gas are water vapor and carbon dioxide.
- Air is 60% heavier than natural gas.
- It has a low flammability range and a high ignition temperature.
- Generally, it is transported through pipes.
- It occurs naturally in the rocks beneath the earth’s surface, in sedimentary rocks that are porous.
Applications
- It is used in vehicles in the form of CNG (Compressed Natural Gas).
- It is also used to manufacture a few chemicals and fertilizers.
- The gas is used as a source of energy for cooking, heating, and electricity generation.
- It is used as chemical feedstock for manufacturing plastic and other commercially important organic chemicals.
- The gas is also useful in the production of fabrics, glass, paint, plastic, steel, etc.
- Hydrogen can also be produced with the help of it.
- Protein-rich animal and fish feed is produced by feeding this gas on a commercial scale.
Hydrogen gas as a future fuel
The main purposes to produce green hydrogen is to add it to the natural gas system, because the natural gas system is seen as a seasonal and large energy storage and necessary for balancing the intermittent electricity production from renewable wind and solar. When hydrogen is mixed with natural gas, the hydrogen content can vary in a wide range. Gas engines have the capability to burn gases with a wide range of hydrogen content, but there are also some challenges with a potentially high fluctuation of the hydrogen content or fast rate of change.
The two technology areas, traffic decarbonisation and long-term energy storage, depend on H2. As H2 will be transported in pipelines, the need for flexible H2-tolerant gas engines will rise.
Power alcohol
Definition :-
When ethyl alcohol is used as fuel internal combustion engine .it is called as power alcohol . combustion engine it is called as power alcohol generally , ethyl alcohol is used as its 5 – 25 percent mixture with petrol.
- India has started mixing 5 percent ethyl alcohol in petrol from year 2003 but countries like brazil use 25 percent .ethyl alcohol in petrol from 1970.
2. Use of ethyl alcohol is petrol reduces our dependence for petrol on foreign reserves by about rs 5000 annum. Further it gives boost to the indigenous manufactures of ethyl alcohol and farmers get better price of their grain produce .
3. Ethyl alcohol is mainly manufactured by fermentation of molasessstretch .carbohydrates on a large scale and cheaply . it can also be obtained from synthesis of gas ( co , H2 ).
Reactions :-
|
4. The starch or sucrose in molasses is converted into ethyl alcohol by fermentation as in above reactions the ethyl alcohol obtained contains 95.5 percent alcohol is separated by use of suitable dehydration agent or by distilling it along with benzene .
Advantages of power alcohol :-
- Ethyl alcohol has very good anti knocking property and its octane number is go , while the octane number of petrol is about 65 ( percent ).
Therefore, addition of ethyl alcohol to petrol increases its octane number.
2. Alcohol has property of absorbing any traces of water if present in petrol.
3. If a specially designed engine with higher compression ratio is used, then the disadvantage of lower c.v of ethyl alcohol can be overcome.
4. Ethyl alcohol contains O atoms which help for complete combustion of power alcohol and the polluting emissions of co , hydrocarbon is reduced largely.
5. Use of ethyl alcohol in petrol reduces our dependence on foreign countries for petrol and saves foreign currency considerably.
6. Power alcohol is cheaper than petrol.
Disadvantages :-
- Ethyl alcohol has c.v 7000cal /gm . use of power alcohol reduces power output up to 35 ( percent )
2. Ethyl alcohol has high surface tension and its atomization especially at lower temperature is difficult causing starting trouble.
3. Ethyl alcohol may undergo oxidation to form acetic acid which corrodes engine parts .
4. Ethyl alcohol obtained by fermentation processes directlycannot be mixed with petrol, but it has to be dehydrated first.
5. Prepare sodium methoxide from sodium metal and methanol .add the sodium methoxide about 2 percent by weight to vegetable oil or fat.
6. Add methanol about 20 percent by volume to the mixture .
7. Heat the mixture with stirring for 30 min
8. Cool and mix sufficient water stir well the glycerol and soap dissolve in water phase .
9. Separate the water insoluble phase from water phase
10. Add antioxidant to the biodiesel to avoid it to become gumany due to oxidation and polymerization.
Biodiesel can be obtained from various vegetable oils like soya beenoil ,palm oil , groundnut oil , cottonseed oil , mustard oil , flower oil , etc. . and also, from the animal fats .
The product is given name biodiesel on account of that it is obtained from biological product and It is biodegradable material.
Advantages of biodiesel:-
Biodiesel can be used as a good fuel for diesel engine but generally it is used as its 20 percent mixture with diesel.
- Biodiesel is cheaper
2. It has high c.v of about 40 kg / gm
3. It is regenerative and environmentally friendly .
4. It does not give out particulate and CO pollutants .
5. It has certain extent of lubricity
6. It uses provided good market to vegetable oils and reduces our dependence on biodiesel onforeign countries , saving currency.
7. It is clean to use biodiesel in diesel engine .
References:-
1. Engineering Chemistry by O.G. Palanna, Tata Magraw Hill Education Pvt. Ltd.
2. Textbook of Engineering Chemistry by Dr. S. S. Dara, Dr. S. S. Umare, S. Chand & Company Ltd.
3. Textbook of Engineering Chemistry by Dr. Sunita Rattan, S. K. Kataria& Sons Publisher