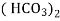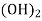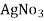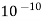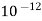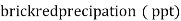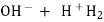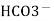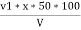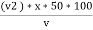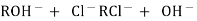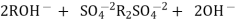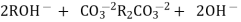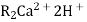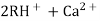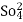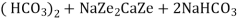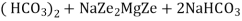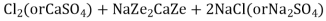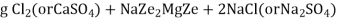UNIT 1
WATER TECHNOLOGY
- Water is covalent compound.
- It contains O and H elements bounded by polar covalent bond.
- Water can form inter molecular hydrogen bond.
- Water has high dielectric constant 78.5 (at 25-degreec) There by possessing good ability to overcome the force of attraction between the oppositely charged ions in ah ionic solid.
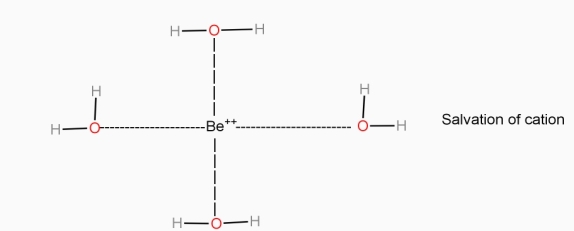
- The polar water molecule can form an envelope around the ions (solvation) and stabilize them. The solvation of ion of a solute is always heat liberating processes.
- The smaller sized ions like
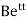 ,
, 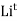 ,
, 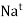 , etc. can be solvated more easily with greater amount of stability but bigger sized ions are solvated with difficulty or lesser stability.
, etc. can be solvated more easily with greater amount of stability but bigger sized ions are solvated with difficulty or lesser stability. - The criteria for dissociation of solute in solvent are ‘ like dissolves like ’ lower molecular weight of solute, high temp, higher pressure ( for gaseous solute ) and ability of solvent to break solute –solute inter – molecular force of attraction or ability to separate the ions in solute.
- ‘like dissolves like’ is meant by the similarities in solute and solvent molecule regarding kinds of atom, nature of bond, structure , etc. . greater the extent of such similarities in solute and solvent molecules higher is the tendency to form solution.
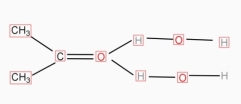
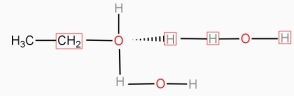
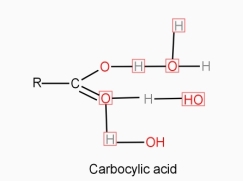
- Molecular solid like urea,sugar,alcohols,carboxylic, acids , acetone , dissolve in water.
- The solute solvent intermolecular force of attraction if is greater than solute – solute in inter molecular force, then the solute is soluble in the solvent.
- Water can break the intermolecular forces of the attraction in the above types of molecular solids, separate the solute molecules from each other and gets dispraised in water.
- Many times the separated solute molecules are solvated by hydrogen bonding.
Ex: -
KETONE
|
5. ‘like dissolve like’ principle is applicable for molecular solids dissolution in water.
- Water has dielectric high constant 78.5 at 25-degree C. Therefore, it can separate the ions and ions in an ionic solids.
- Certain number of H2O molecules from weak bond with the separated ions and form an envelope of solvent molecules.
- The solvated ions are prevented form their reunion such solvation offers stability to ions in solution and always liberates certain amount of solvation energy.
|
4. However, all ionic solids are not soluble in water. solubility of an ionic solid in water is only when solution energy.
5. Bigger sized ions have lower solutions energy than their lactice energy and therefore they are not water soluble.
e.g.: -agcl,phs,kcl, e.t.c .
6. various ionic solid Nacl,Kcl, Caso4,e.t.c. have higher solution and are water soluble.
- The gases like SO2,CO2, NO2 Hcl O2 e.t.c. have certain extent of similarities with water molecules regarding nature of atoms, nature of bonds, polarity in bonds,structure,e.t.c. and therefore, they are more or less soluble in water particularly at lower temperature.
2. Because a large number of substances dissolve in water, water is called as universal solvent. chemical analysis of water. Generally analysis is done for known small quantity of water sample. These constituents are determine under the heading.
- Hardness
- Chloride content
- Alkalinity
- Sulphate content
- Silica content
Hardness can be defined as a soap consuming capacity of water sample. soaps are sodium salts of fatty acids like oleic acid, palmetic acid and stearic acid. they dissolve readily .in water to form lather due to which it has cleansing property.
(calciumstearate)
|
- Temporary hardness ( carbonate) :- v
Mg ( Bicarbonates)
|
II. Permanent hardness :-
- The term permanent hardness ornon carbonate is the term applied to the hardness caused by dissolved chlorides , nitrates and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- Sum of temporary and permanent hardness is referred to as total hardness.
III. Alkaline or carbonate and non – alkaline or non – carbonate hardness :-
- Like all carbonate and bicarbonate , calcium and magnesium carbonate and bicarbonate are alkaline.
- Then hardness due to the carbonate and bicarbonate is called alkaline hardness or carbonate hardness.
- The alkalinity can be measures by titration with standard mineral acid using methyl orange and or phenol phthalein as an indicator.
4. As the sulphate and chloride are neutral salts , the hardness caused by presence of calcium and magnesium sulphate , chlorides and nitrates is termed as non alkaline hardness or non carbonate hardness.
IV. Total hardness :-
The hardness due to all hardness causing salts , known as total hardness.
Total hardness = temporary + permanent
Estimation of hardness :-
Hardness of weather can be determined by two methods.
1) Soap solution method :-
- Total hardness of water can be determined by titrating a fixed volume of water sample (100ml) against standard alcoholic soap solution.
- Appearance of stable lather which persists for two minutes is the end point of titration.
- In the beginning sodium soap will precipitate all the hardness causing metal ions in the form of their soap (card) and then it will form free lather.
- If same water sample is boiled for 30minutes and then titrated against same soap solution the titration reading corresponds to permanent hardness.
- The difference between two measurements corresponds to the temporary hardness of water.
- Hardness of water can be determined more accurately by EDTA method.
- In this method 100ml of water sample is taken in titration flask to this 3ml of buffer an 08 ph 10 is added.
- Then it is titrated against 0.01m EDTA using electron black T as an indicator.
- At the end point wine red color changes to blue color.
- From barette reading , total hardness of water sample can be calculated using the formula.
1000ml of 1MEDTA = Caco3 = 100 get Caco3
6. Suppose barrette reading is xml for 100 ml of water sample. Then for one liter of sample 10x ml EDTA is required.
As ,
100 ml 1MEDTA = Caco3 = 100 get Caco3
1 ml 0.01 EDTA = 1 mg Caco3
10 x ml 0.01m EDTA = 10 x mg Caco3
7. Hardness can be expressed as mg of Caco3 present in 10 mg ( 1 liter ) of water i.e. ppm .
Hardness of such water sample will be 10 x ppm
8. If titration is carried out after boiling the water sample for 30 min the reading will correspond to permanent hardness corresponds to temporary hardness of water sample.
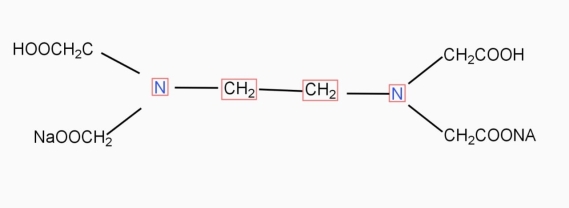
9. Ethylene- diamineteracetic acid (EDTA) IS PRACTICALLY Insoluble in water it is represented as H4Y.
|
INaqueous solution ionises as
Na2H2Y  2Na+ + H2Y2-
2Na+ + H2Y2-
It forms 1:1 complex with Ca++ and Mg++ metal ions present in water sample when indicators is added to water sample colored ( red ) metal indicator complex is formed
When this is titrated with EDTA solution
H2Y²- ions react with Ca++ or Mg++ ions from metal indicators complex because these two have more affinity towards EDTA. So more stable metal EDTA complex is formed .at the same time HIn² -ve ions are set free ( blue ).
So, at the end point color changes from red to blue.
- Parts of per million (ppm) is the parts of the calcium carbonate equivalent hardness per 10 raise to 6 parts of water i.e. 1 ppm = 1 part of
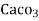 eq hardness in
eq hardness in 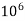 parts of water .
parts of water . - Milligram per liter is the number of milligram of
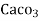 equivalent hardness present per liter of water.
equivalent hardness present per liter of water.
Thus,
1 mg/l = 1 mg of 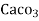 eq. hardness of 1L of water .
eq. hardness of 1L of water .
But 1 L OF Water weighs.
= 1 kg = 1000 g = 1000*1000 mg = 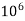 mg
mg
Therefore,
1mg/l = 1 mg of 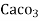 eq. per
eq. per 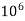 mg of water.
mg of water.
= 1 part of 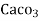 eq. per
eq. per 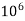 mg of water
mg of water
= 1 part of 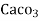 eq. per
eq. per 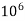 parts of water
parts of water
=1 ppm
3. Clarke’s degree is number of grains (1/7000lb) of 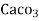 equivalent hardness per gallon (10lb) of water. Or it is parts of
equivalent hardness per gallon (10lb) of water. Or it is parts of 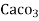 equivalent hardness per 70000 parts of water.
equivalent hardness per 70000 parts of water.
Thus,
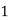 ° clark = 1 grain of
° clark = 1 grain of 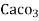 eq. hardness per gallon of water.
eq. hardness per gallon of water.
 1° c l = 1 part of
1° c l = 1 part of 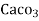 eq. hardness per 70000 parts of water .
eq. hardness per 70000 parts of water .
4. Degree French (°fr) is the part of 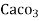 equivalent hardness per
equivalent hardness per 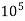 part of water .
part of water .
Thus,
1°fr = 1 part of 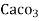 hardness eq. per
hardness eq. per 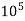 parts of water.
parts of water.
5. Milli equivalent per liter (meq / L )is the number of milli equivalents of hardness present per liter.
Thus,
1meq/L = 1 meq of 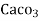 per L of water
per L of water
= 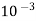 * 50 g of
* 50 g of 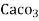 eq. per liter.
eq. per liter.
= 50 mg of 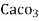 eq per liter.
eq per liter.
= 50 mg / l of 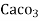 eq = 50 ppm.
eq = 50 ppm.
Relation between various units of hardness
- 1 ppm = 1 mg / L 0.1° fr = 0.07° Cl = 0.02 meq/L
- 1 mg/L = 1 ppm = 0.1° fr = 0.07° cl = 0.02 meq / L
- 1°cl = 1.433° fr = 14.3 ppm = 14.3 mg / L = 0.286 meq / L
- 1° fr = 10 ppm = 10 mg/ L = 0.7° cl = 0.2 meq / L
- 1 meq/ l = 50 mg/ l = 50ppm = 5° fr = 0.35° cl
Chloride content : -
Chloride ions amount in water sample ( bymoho’s method)
- Under ground and surface water are rich with cl- in the form of Nacl ,Kcl , e.t.c .
- Their quantity over 250 mgl liter imparts bad taste to water. Mgcl2 , Cacl2 cause hardness and being the salts of weak base strong acid are harmful for industry and boiler use .
Theory
3. When Ag + ions are added to the mixture container of an indicator Cro4 there is first formation of Agcl , although Agcl has lesser solubility product than Ag2Cro4. 4. The reason for why Ag2Cro4 precipitate formation requires excess concentration of Ag++ to exceed its ksp.
|
Procedure :-
- Take 50 ml of a chloride water sample in a conical flask and add a pinch of caco3 to neutralize the water if it is acidic.
- Add few drops of a potassium chromatic indicator solution into the water and slowly add Agno3 solution( z molarity ) from burette.
- Note the end point when permanent reddish tinge is obtained .let the burette reading by Y ml .
Calculations :-
 volume of
volume of 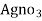 for 50 ml water sample = Y ml
for 50 ml water sample = Y ml
∴ volume of 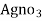 for 1000 ml water sample 1000/50 * y ml
for 1000 ml water sample 1000/50 * y ml
= 20 y ml
∴ 1000 ml 1 m 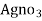 = 35.5 * 1000 mg cl
= 35.5 * 1000 mg cl
∴ 20 y ml 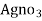 =
= 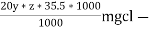
= 20yz * 35.5 mg cl- / liter
A natural water may be alkaline due to presence of hydroxide bicarbonates and carbonates compound dissolve in water.
Hydroxides 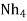 OH ,NaOH
OH ,NaOH
Bicarbonates Ca (Hco3)2
Carbonates Mgco3 , Feco3
Hydroxides and carbonates and stronger bases than bicarbonates.
- When an alkaline water is titrated with a strong acid first all OH get neutralized then all the caco3 – ions are half neutralized + OHCo3- .
- Till this stage ,ph of mixture decreases to about 8.2 and completion of this stage is indicated by change in color of phenolphthalein.
- On continued addition of acid during titration all the HCO3 in the titration mixture ( produce by half neutralization of CO3 and present from beginning ) get neutralized and completion of this stage is indicated by methyl orange color change at about3.7 ph.
|
Procedure :-
The alkalineties due to the three type of ions can be easily determined by neutralisation titration.
- Take V ml ( generally 25 ml ) of the alkaline water in conical flask and add 2 drops of phenopthalein indicator in it .
- Titrate this sample against standard strong acid solution ( x n ) from burette till pin k colour changes to colourless . klet the burette be V 1 ml .
- Add few drops of methyl orange indicator into the same titrarting mixture changes to orange .
Note the burette reading as V 2 ml ( from initial )
Calculations :- P = phenolphthalein alkalinity = = PPM Caco3 equivalent M = methyl orange alkalinity = total alkalinity =
|
The possible combinatuions of alkalinites in water are:-
- Only OH-
- Only HCO3-
- ONLY CO3-
- OH- and CO3 – Togther
Formula :-
Hardness of water by EDTA
1) When standard hard water is used
Total hardness of water sample = 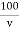 .
. 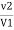 . Mg. CaCo3
. Mg. CaCo3
Where,
M = mgCaco3 in titrated standard hard water
V1 = EDTA volume for titrated standard hard water
V2 = EDTA volume for V ml water sample
2) When EDTA of known molarity used
Total hardness of water sample =  *z*
*z*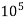 MgCo3
MgCo3
Where,
Y = EDTA volume for V ml of water sample
Z = molarity of EDTA solution
Chloride ions in water sample :-
cl- quantity = 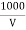 yz (35.5) mgel/L
yz (35.5) mgel/L
Where,
Y = volume of AgNo3
Z = molarity of AgNo3
Alkalinites in water sample
Phenolphthalien alkalinty
P = 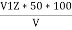 PPM Caco3 equivalent
PPM Caco3 equivalent
Methyl orange alkalinity
M = 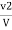 * z * 50 * 1000 ppm
* z * 50 * 1000 ppm
Where ,
V1 = volume of acid for V ml water sample in titraton with use of phenolphthalein indicator.
V2 = volume of acid for V ml water sample in the continued titration using methyl orange indicator.
Z = normally of acid in barette.
In steam geeneration and to increase the lige of the boiler .
For the purpose the feed water is treated well externally ( by processes like ion exchange , zoolite , soda lime ) and internally ( by processes like phosphate , conditioning , hydrazine , tretament )depending upon the operating pressure of boiler , the feed water should satisfy the following requirement.
Type of boiler | Permitted hardness of feed water |
Low pressure below 15kg/cm² | 25 – 50 ppm caco3 equivalent |
Medium pressure 15 – 30 kg/cm² | 10 – 25 ppm caco3 equivalent |
High pressure greater than 30 kg/cm² | 0 – 10 ppm caco3 equivalent |
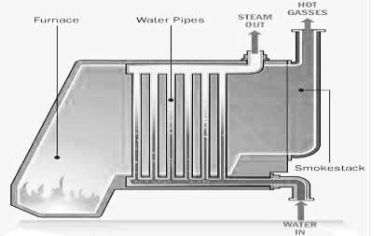
- The most important use of water as enginnering material is the steam generation .
- Steam is required for power generation in thermal power stations for uniform and controlled heating of reactors for steam engine e.t.c .
- Different design of boilers ( water tube, vertical , e.t.c ) are used in making steam of different pressure and temprature.
- Water of minimum hardness 9 below 10 ppm is used for high pressure steam boilers ) and maximum purity ( total dissolved solids below 25 mg / lit) is good for steam generation .
- Water of higher hardness and other imparites is treated well before . fedding it in boiler.
|
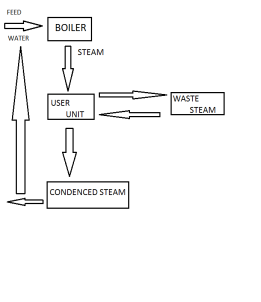
Steam generation can be economized by recircuit calculating the waste steam on reheating and recircualting the steam condensalte to boiler from the steam generation.
|
Major boiler problems due to use of unsutiable water are :-
- Corrison
- Priming and foaming
- Sludges and scales formation
- Caustic embittlement
Out of these boiler troubles the corrison and seals formation are much serve and requires atmost care.
- Corrison in boiler tube ( heat exchange tube is ) mainly due to presence of dissolved gaseous like CO2 , O2 in water and due to presence of acids in water presence of Mgcl2 directly attacks the boiler metal and corrodes it.
- Corrison is the destruction of metal by chemical or electro chemical attack of the envoriment . in boilers if feed water contains dissolved oxyegen when wter is boiled O2 is set free . This metal of boiled 2fe+2H2O+O2 - - - - - - - - - 2FE(OH)2 - - - - - - - - Fe ( OH ) 2 -------- Fe2O3.H20 Formation of rust by adding calculated quantity of hydracine to water oxygen from water can be removed
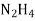 +O2 -------------------
+O2 -------------------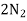 +
+ 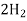 O
O
3. In low pressure boilers sodium sulphaite is used for removing oxygen.
4. If water contains dissolved co2 it form carbonic acid with water this acid produces local corrison called as pitting .
5. Carbon dioxide and oxygen are to be removed from feed water by deaeration . co2 can be removed by lime treatment . as an internal treatment . co2 is converted to ammonium carbonate by addition of ammonia.
6. Excess ammonia evaporates in boiler along with steam and gas in condenser.
7. Acidity of water can be decreased by adding calculated quantity of NaOH to it so that Ph of water will be in between 8 and 9 ph units.
Processes :-
- When a boiler is steaming (i.e producing steam ) rapidly some particles of liquid water are carried along with the steam . this processes of wet stram formation is called priming.
- Priming is caused by :-
- The presence of large amount of dissolved solids
- High steam velocites
- Sudden boiling
- Improper boiler design
- Sudden increase in steam production rate
3. Foaming :-
- It is the production of persistent foam or bubbles in boilers which do not break easily.
- Foaming is due to presence of substance like oils ( which greatly reduce the surface tension of water )
4. Priming and foaming usually occur togther. They are objectionable because:-
- Dissolved salts in boiler water are carried by the wet steam to super – heater and turbine blades where they get deposit as water evaporates . this deposite reduce their efficiency.
- Dissolved salts may entire in the parts of other machinery where steam is being used there by decreasing the life of the machinery.
- Actual height of water column Cnnot be judge property there by making the maintance of boiler pressure becomes difficult.
Priming can be avoided by :-
- Fitting mechinical steam puritires
- Avoiding rapid change in steaming rate
- Maintaning low water levels in boilers
- Efficent softening and filteration of boiler – feed water
Foaming can be avoided by ;-
- Adding anti – foaming chemical like castor , oil or
- Removing oil from boilerr water by adding compounds like sodium aluminate.
- It is a type of corrison caused by high concentration of sodium hydroxide in boiler water sodium carbonate hydroxide in the boiler and extent of hydrolysis increases with temprature.
Na2CO3 + H2O ----------------- 2Na.OH + CO2
2. Sodium hydroxide reacts with metal ion to form ion to form iron oxide and hydrogen.
3. When metral oxide coating cracks, chemical attack continues into metal along grain boundaries . through the cracks , water flows , wwhen it evaporates sodium hydroxide is left there.
4. This attack iron of boiler.
5. This type of corrision of boiler parts particularly at stress parts caused due to chemical of caustic soda ( NaOH) Is called as embrittlement.
6. During water softening by soda – limes , processes Na2CO3 is added to water as precipitant . caustic embrittlement can be prevented by addition lignins or tannos which help in blocking of hair cracks.
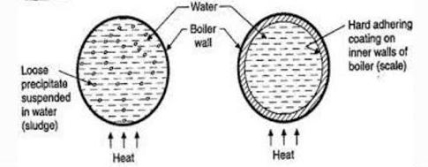
Sludge formation , scale formation
- In boiler water evaporates continously and the concentration of salts left behind goes on increasing . After the saturation point they get precipitated.
- If the precipitate remains in boiler tube as loose and slimy matter is called sludge.
- If some of the precipitated matter adhers strongly and forms strong bad conducting layer on their inner side of boiler tube , then it is known as scale.
Sludge :-
( formation of sludge )
- The loose slimy mass of salts precipitated in boiler water is the sludge.
- They are generally formed at cooler portion of boiler and they loosely deposit in the parts of boiler tube where flow rate is slow e.g vlves bends
- Sludges are easy to remove by using brushes detergent solutions blow down opreation e.t.c .
Disadvanatages of sludges :-
- They tend to waste some portion of heat.
- Edcessive sludge formation distrub working of boiler and sometimes may choke up the pipe .
Prevention of sludges :-
- Use of water contaning very low quantity of total disolved solids.
- Frequently making blow down opreation i.e replacing salts concentrated water with fresh water.
A] formation of scales :-
Scale is the hard and strongly adhered coating to the inner surface of boiler and it is a bad conductor of heat. It is the main source of boiler trouble.
It is caused due to :-
- Decomposition of bicarbonates :-
At high temprature bicarbonates decompose into sticky water insolube material.
Ca ( HcO3)2 ----------------- CaCO3 + H2O + CO2
Mg ( HCo3)2 - - -------------- Mg (Oh)2 + 2CO2
2. Hydrolsis of magnesium salts :-
At higher temprature magnesium salts undergo hydrolysis.
MgCl2 + 2H2O ---------------------- mg ( OH)2 + 2HCl
3. Presence of silica :-
The source of ssilica is ( form) from sand and filter .silica may be in the form of colloidal particles. And it can be deposite as calcium silicate or magnesium silicate as firmly adhering materials.
4. Decreased solublity of CaSO4 :-
CaSo4 has lesser solublity at higher temprature hence at high temprature CaSo4 present in boiler feed water will precipitate as hard scale forming materials.
|
B] Disadvantages of scale :-
1. waste of fuel :-
Scales are bad conductors of heat and resultes in the reduction of heat transfer to the boiler . higher the thickness of scale greater than the wastage of fuel there by . it has been reported that 0.25 cm . scale would increase fuel consuption by aboyut 2 to 3 percent.
Thickness of scale | 0.325 mm | 0.625mm | 1.25mm | 2.5 mm |
Wastage of fuel | 10 percent | 15 percent | 50 percent | 80 ercent |
3. over heating of boiler :-
Scale being pooe conducter of heat it reduces transfer of heat from boiler to boiler water . to keep the required steam pressure we need to overheat the boiler .
4. boiler saftey :-
due to the scale formation the overheating of boiler is done in order to maintain constant stream supply with required pressure . this overheating makes boiler metal to become soft and weak . this cause distortation of boiler tube and becomes dangerous in morden high pressure boiler .
5. Danger of Explosion :-
When thick scale cracks due to uneven expansions the water come suddenly suddenly in contact with the overhead boiler metal. This cause large amount of steam formation suddenly and sudden high pressure is developed . due to sudden high pressure is devloped . due to sudden high pressure the softer boiler metal may burst with explosion.
C] Removal of scales :-
Scales are removeed from time by different ways.
- By use of suitable chemicals the scale can be dissolved and removed.
- Use of scraper or wire brush for thin scales to remove.
- Thick s ales may br removed by hammer and chisle.
- The thermal shocks technique is used to remove hard brittle scale . in this method empty boiler is heated and cooled by cold water suddenly . while sudden cooling the contracting boiler metal excerts pressure on scale to crack them.
- Blow down opreation used if scales are loosely adhering.
D] prevention of scales :-
It is better to minimize scales formation and reduce the problems in steam generation.
- Use of softened water.
- Adding sodium phosphate to the water
- Frequent blow down operations to remove the scales when they are thin.
- Adding sodium aluminate which can trap the scale forming particles.
- Adding organic chemicals like tannin which forms coating on the scale forming particles . this matter becomes easily removable by blow down operation.
DIFFRENCE BETWEEN SLUDGE AND SCALE
sludge | scale |
Sludge is loose deposite of slimy matter | Scale is hard coating |
Sludge is less adherent on boiler metal and can br removed by brushes detergents | Scale is strongly adhered to boiler metal and difficult to remove |
Heat transfer to boiler water is affected slightly | Being bad conductor heat transfer to boiler water is affected largely |
Sludge form at coller parts and flow rate is low | Scale is formed at hotter parts |
Sludge may lead to choking | Scale may lead to bluging of metal tube its bursting explosion |
Softening methods :-
Water used for industrial purpose ( such as for steam generation) should be sufficiently pure it should be therfore be freed from hardness producing salts before put to use the processes of removing hardness producing salts from water is known as softening water is known as softening of water . in industry main three methods employed for softening of water are
- Ion exchange or de ionisation
- Zeolite
- Lime soda processes
- Ion exchange or de ionization or de mineralization ion exchange resins are insoluble cross linked long chain organic polymers with a microporous structure and the functional group attached to the chains are responsible for the ion exchange properties resins contaning acidic function groups are capable of exchaning their anions with other anions which comes in their contact the ion exchange resins may be classified as :-
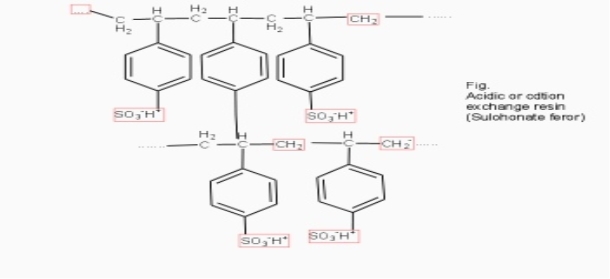
Cation exchange resins ( RH+) :-
They are mainly styrene divinly benzene co-polymers which on sulphonation or carbonoxylation become capable to exchange their hydrogen ions with the cations in the water.
|
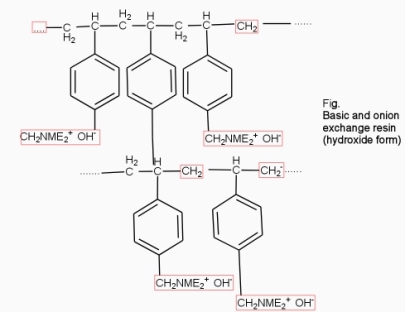
2. Anion exchange resins :-
They are styrene – divinly benzene or amine fermaldehyde which contain amino or quaternary ammonium or4 quaternary phosphonium or tertiary sulphonium group as an integral part of the resin matrix.these after treatment with dilute NaOH solution become capable to exchange their OH- anions with anions in water .
|
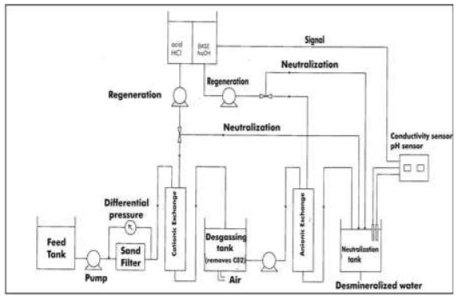
Processes :-
The hard water is passed first through cation exchange column which removes all the cations like Ca²+ , Mg² + e.t.c . from it and equivalent amount of H+ ions are released from this column Water
2RH+ + Ca².+ ---------------------- R2Ca2+ + 2H 2RH+ Mg2+ --------------------------- R2Mg2+ 2H+
After cation exchange column the hard water passed through anion like cl- . present in the water and equivalent amount of OH- ions are released from this column to water,
H+ And OH – ions ( released from cation exchange and anion exchange columns respectively ) get combined to produce water molecule. H+ + OH - -- ----------------- H2O Thus, The water coming out form the exchanger is free from cations as well as an ions . Ion free water is known as de mineralizes water.
Regeneration :- When capacities of cation and anion exchangers to exchange H+ and OH- ions respectively . Are lost they are then said to be exhausted. The exhausted cation exchange column is regenerated by passing a solution of dil.HclH2SO4. The regeneration can be represented as
The column is washed with deionized water and washing ( which contains Ca2+ and Cl2- ions ) is passed to sink or drain. The exhausted anion exchange column is regenerated by passing a solution of dil. NaOH.
The column is washed with deionized water and washing ( which contains NA+ and The regenerated ion exchange ion exchange resins are then used again.
|
Advantages :-
- The processes can be used to soften highly acidic or alkaline waters.
- It produces water of very low hardness ( say 2 ppm ) so it is very good for treating water for use in high pressure boilers.
Disadvantages :-
- The equipment is costly and more expensive chemicals are needed.
- If water contains turbidity, then the out- put of the processes is reduced .the turbidity must be below 10 ppm if it is more it has to be removed first by filtration.
Mixed – bed deionizer :-
Consist essentially of a single cylinder containing and intimate mixture of hydrogen exchanger and strongly basic an ion exchanges when water is passed through this bed it comes in contact a number of times with the two kinds of exchanger alternative consequently the net effect of mixed bed exchange is equivalent to passing water through a series of several cation and anion exchangers .the outgoing water from the mixed – bed contains even less than 1 ppm of dissolved salts.
Regeneration :-
When the resins are exhausted the mixed bed is back – washed ( by forcing water in the upward direction ) when the lighter anion exchanger get displaced to form an upper layer above the heavier cation exchanger ( shown in fig ) thereafter the anion exchanger is regenerated by passing caustic soda solution from the top and then rinsed the lower cation exchanger bed is then regenerated by the H2SO4 solution treatment and then mixed again by forcing compressed air . the bed is ready for use again.
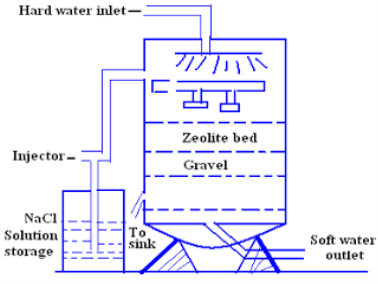
Chemical structure of sodium zeolite may be represented as Na2O. AL2O3. XSIO2.Y.H2O where x=2-10 and Y=2-6 zeolite is hydrated sodium aluminum silicate capable of exchanging reversibility its sodium ions for hardness producing ions in water zeolites are known as zeolite. zeolite are also known as permutits.
Zeolite are of two types
- Natural zeolites :- are non – porous for example natrolite Na2O3.
- Synthetic zeolites: - they are prepared by heating together china clay and soda ash. Such zeolite possesses higher exchange capacity per unit weight than natural zeolites.
Processes :-
For softening of water by zeolite processes hard water is percolated at a specified rate through a bed of zeolite kept in a cylinder (shown in fig.)
The hardness causing ion are retained by the zeolite as caze and MgZe while the out-going water contain sodium salts reaction take place during the softening processes are
Ca Mg Ca M
|
Regeneration-
After some time, the zeolite is completely converted into calcium and magnesium zeolite and it ceases to soften water,i.e. it gets exhausted.
At this stage the supply of hard water is stopped and the exhausted zeolite is reclaimed by treating the bed with a concentrated ( 10 percent ) brine ( NaCl ) solution.
The washings ( containing CaCl2 and MgCl2 ) are led to drain and the regenerated zeolite bed thus obtained is used again for softening purpose.
Limitations of zeolite processes :-
- If the supply of water is turbid the suspended matter must be removed (by coagulation, factorization, etc.) before the water is admitted to the zeolite bed , otherwise the turbidity will clog the processes of zeolite bed there by anking it inactive.
- If water contains large quantities of colored ions such as Mn2+ they must be removed first because these ions produce manganese and iron zeolite which cannot be easily regenerated.
- Mineral acids if present in water destroy the zeolite bed and therefore they must be neutralized with soda before admitting the water to the zeolite softening plant.
Advantages of zeolite processes:-
- It removes the hardness almost completely and water of about 10 ppm hardness is produced
- The equipment used is compact occupying a small space
- No impurities are precipitated so there is no danger of sludge formation in the treated water at later stage
- The processes automatically adjust itself for variation in hardness of incoming water
- It is quite clean
- It requires less time for softening
- It requires less skill for maintenance as well as operation
Demineralization process is the modern industrial water softening process. By this process the, It can be passible remove of hardness as well as remove of all dissolve salts i.e;. FeCO3, CaCl2 .
We can also say that demineralization or deionization is the process of removing the dissolved ionized solids from water by ion exchange.
The major portions of total dissolved solids (TDS) are mineral salts, such as calcium bicarbonate, magnesium sulfate, and sodium chloride.
Mineral salts are composed of cations and anions. Since deionization requires the removal of all ions, both the negatively charged anions and the positively charged cations, then materials capable of attracting both are required.
These materials are known as cation and anion exchange resins.
|
References:-
1. Engineering Chemistry by O .G. Palanna, Tata Magraw Hill Education Pvt. Ltd.
2. Textbook of Engineering Chemistry by Dr. S. S. Dara, Dr. S. S. Umare, S. Chand & Company Ltd.
3. Textbook of Engineering Chemistry by Dr.Sunita Rattan, S. K. Kataria& Sons Publisher