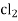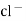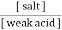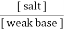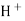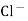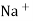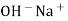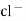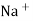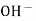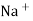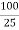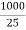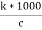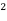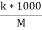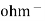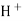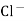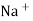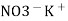UNIT 2
INSTRUMENTAL METHODS OF ANALYSIS
2.1 Introduction: Types of reference electrode
Types of reference electrode
Definition:-
Reference electrode is defined as the electrode which has stable and reproducible potential and completes the cell acting as half-cell.
Purpose of reference electrode is to complete the cell and provide stable potential against which indicator electrode is compared and measured . the reference electrode is designed to produce the same potential no matter in which solution it is placed.
Following electrodes are commonly used as reference electrodes:-
- Calomel electrode
- Standard hydrogen electrode
- Silver- silver chloride electrode
- Glass electrode
- Quinty drone electrode
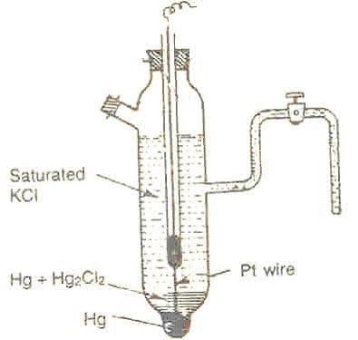
Calomel electrode :-
This is most widely used reference electrode.
Construction :-
- Calomel electrode consists of a narrow glass tube at the bottom of which is liquid mercury , above it is a paste of Hg – Hg2 and remaining portion of glass tube is filled with 1N or 0.1N solution or saturated solution of Kcl.
- A platinum wire dipping into the mercury layer is used to make electrical contact.
- A dipping type of calomel electrode has tapering lower and the bottom is porous .
- In some calomel electrodes there is side tube filled with gel for acting as salt bridge and making electrical contact.
Calomel electrode is represented by :-
Hg 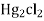 (s)
(s) 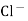 sat.
sat.
Reaction :-

 If calomel electrode acts as athode reaction is
If calomel electrode acts as athode reaction is
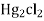 (s) +
(s) + 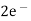 2Hg +
2Hg + 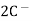
And if it acts as an anode then the reverse reaction.
Potential :-
The reduction electrode potential is given by
Ecal = E° - 0.0591 log [ 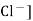
( if calomel electrode is connected to standard hydrogen , electrode , potential being zero , the emf of such cell is equal to potential of calomel electrode)
Potential of the calomel depends on 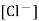
Kel conc. | 0.1N | 1 N | STANDARD |
E° | 0.3334 Volts | 0.281 volt | 0.2422 V |
Demerits of calomel :-
- It should not be used above 50°c
- It involves poisons Hg and Hg2Cl2
|
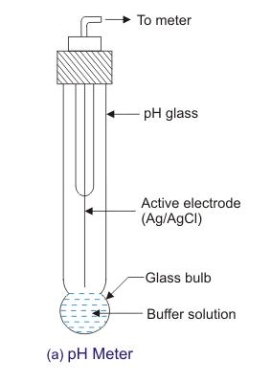
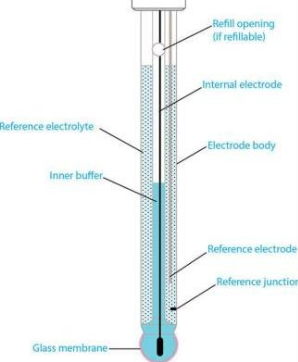
Glass electrode :-
Principle :-
When two solutions of different [h+] are separated by a thin glass membrane a potential difference is developed the two surfaces of membrane . the potential difference in [H+] of the two solutions.
Ion exchanger
i.e. exchange of Na+ of glass with H+ of solution.
Construction :-
- A glass electrode is a long glass tube with thin walled glass membrane bulb at the bottom.
- A.pt wire or A gel coated Ag wire is dipped in the 0.1 M Hcl solution in the Bulb . the glass electrode is representing as,
Pt 0.1 M Hcl 1glass
Potential 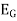 =
= 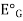 +0.0591 ph
+0.0591 ph
Therefore ,
E.g. is the potential of glass electrode in a solution of known ph.
Glass electrode :-
|
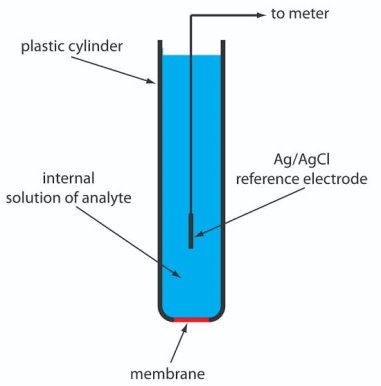
Ion selective Electrodes
Definition:-
A membrane of a half cell is sensitive to particular ion in solution and ion exchange take place between the membrane electrode and the solution containing specific ions and develops a potential which depends upon the concentration of that ion.
The potential developed at the ion sensitive sensor is a measure of concentration of the ions of interest. E.g. . H+ sensor ( glass electrode) Ca++ sensor ( calcium ion selective electrode ) etc.
Determination PH of solution :-
- A glass electrode is coupled with calomel electrode to determine the ph. of solution .
2. The electrode is dipped into the solution of unknown Ph.
3. The cell is represented as
Pt. | Hg.Hg |
| Glass electrode |
EmF of the cell is measured (Ecell)
E cell = E cal - E g = 0.2422 - E°g + 0.0591pt)
Therefore,
PH = 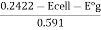
E°g is the potential of electrode when glass electrode is in contact of solution of known PH.
Advantages :-
- Glass electrode is advantages as it is simple to use
- Gives accurate and quick result and the electrode is not getting poisoned.
- Equilibrium is easily obtained .
- It is stable electrode to use oxidizing and reducing agents.
- It is portable and compact
- Chemical sensors depend on ions
- It also measures cone of ions
- It is used in potentiometric titration.
Hence the ion selective membranes can also be called ass chemical sensors.
These electrodes not only detect but also measure the concentration of that ion in a solution.
Types of ion selective electrodes:-
- Glass membrane electrodes :-
- These electrodes are formed by doping .often sio2 with various chemicals.
- The most common such electrode is the H+ sensitive electrode or PH electrode.
- The H+ from solution get exchanged with LI+ from solution get exchanged with Li+ in the glass.
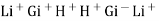
Thus, different glass composition can be made to measure Na+ , Ag+, K+, NH4
|
- On immersing glass electrode in a solution , a hydrated gel layer formed cause swelling of the membrane . the ion exchange processes take place in the gel layer of the glass membrane , generates a phase boundary potential given by ,
E gl. = E° gl. + 0.0591 log 10 [ m+n ]
E°gl = standard electrode potential
[ M+n] = concentration of the ion of interest in solution.
Solid state electrodes :-
In the non-glass solid state sensors ionic conducting membrane are used . e.g.
- Homogenous electrode of finely divided crystalline material like LaF3 for determination of F- in water
- Heterogeneous electrode consisting solid crystalline material incorporated with polymer like PvC or Silicone.
|
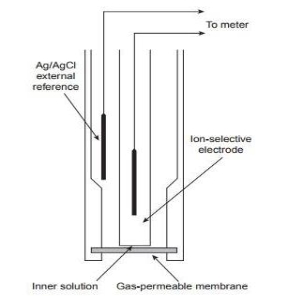
Gas sensing electrodes :-
- These electrodes are useful to analyze gases such as NH3 , NO2 , SO2 , CO2.
- A nitrate ion responsive electrode is for NO2 while a sulfide ion selective electrode is for H2s
|
3. The microporous membrane is hydrophobic made from polypropylene or any other fluorocarbon which allows only dissolved gases to pass through
4. The electrodes Ag / Agel and glass PH – electrode is dipped in the inner solution.
5. Area of membrane being small and volume of liquid being less it reaches equilibrium with test solution rapidly.
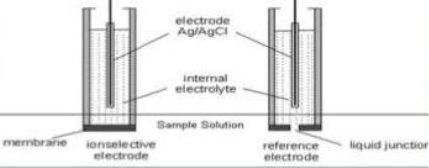
Liquid membrane electrode :-
- Liquid ion exchange membranes have functional groups to form co – ordination.
- The inner solution containing known concentration of the ion is placed in a glass tube fitted with Ag / Agcl electrodes selective to it
- The outer aqueous solution by liquid is separated from the inner solution by liquid ion exchange membrane.
- The membrane seals the bottom of the electrode vessel.
- The outer compartment contains the organic liquid ion exchanger.
- The membrane is replaceable.
- The boundary potentials across the two-solution membrane interface is functionally dependent on the ion activity and is fixed at a constant temperature.
|
Enzyme electrodes :-
These are similar to glass electrode . they are actually carbonate ion selective glass electrodes which sense Co2 . immobilized enzyme like L – arginine or L- sine carboxylase is used in these electrodes. They are selective for amino acids that generate CO2.
Ph is defined as the negative logarithm of hydrogen ion concentration and mathematically expressed as, PH = - log [ H+] in moles per liter The PH concept is very convenient for expressing hydrogen ion concentration . it was introduced by Sorensen in 1909
Buffer solution :- Buffer solution is the solution which resists change in PH ( even ) i.e. with addition of a small amount of an acid or base to a buffer solution. There are two common types of buffer solution.
A] A weak acid along with a salt of weak acid with strong . this is acidic buffer E.g. :-
B] weak base and its salt with a strong acid. This is basic buffer E.g. :- NH4OH + NH4cl
Preparation of buffer solution :- There are two principal method for preparing buffers
2. Both components are obtained from a prescribed amount of only one component with second component being produced by a specified amount of strong acid or strong base to yield the desired ratio. From the Henderson equation , buffer solution can be prepared , P°H of acidic buffer = PKa + log p°H of basic buffer = PKb + log
properties of buffer solution :-
PH metric Titration
Mixture of acids vs strong base:- Let us consider titration of strong acid ( HCl ) and weak acid ( CH3COOH ) mixture against strong base ( NaOH ) . By titration against standard alkali using PH meter . PH metric titration does not require indicators.
Theory :-
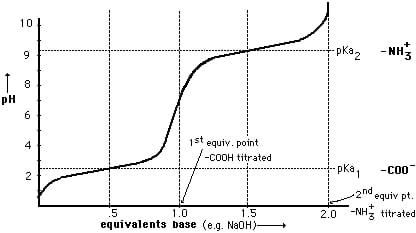
Near the end point there is sudden increase in PH at PH about 3.7 After Hcl completely neutralized , neutralization of CH3COOH begins , there is formation salt. Up to stage of complete neutralization of CH3COOH the titration flask contains CH3COOH + CH3COONa . i.e weak acid and its salt. This mixture acts as buffer solution . The PH of titration mixture also remains constant even through NaOH added from burette
At the end point i.e complete neutralization of CH3COOH , PH increase suddenly , at PH about 8.3 , after this end point , PH increase increased of alkali addition from burette but slowly.
Procedure :-
Part :- 1 calibration ( standardization) of PH meter:- A digital PH meter is calibrated as below
Do not disturb the standardize knob throughout experiment.
Part 2:- Titration of mixture of acids :-
Calculations :- Plot a graph of PH of titration mixture ml of NaOH added and note the first and second equivalent point.
TITRATION CURVE
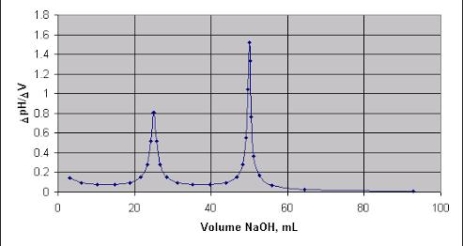
DIFFERENTIAL CURVE PH is the change in PH due to addition of V ml NaOHat every ml NaOH added. First equivalence point V1 ml corresponds to neutralization of Hcl . second equivalence point V2 ml ( from zero ) corresponds to total neutral ofbhclvs CH3COOH . Therefore , Hcl quantity Hcl in 25 ml acid mixture = V1 ml NaOH Hcl in 100 ml acid mixture = = 40 V1 ml NaOH ( Let normality of NaOH be Z) then 1 ml 1 N NaOH = 36.5 mg Hcl 40 V1 ml ‘ z ’ N NaOH = 40 * V1 * Z * 36.5 Mg Hcl Per liter Therefore, CH3COOH in 1000 ml acid mixture = = 40 ( v2 – v1 ) ml NaOH As 1ml 1N NaOH = 60Mg acetic acid
|
Conductometry:-
Terms involved
The conductance is the property of the conductor ( metallic as well as electrolytic) which allow facilitates the flow of electricity through it is equal to their reciprocal of resistance i.e conductance = the conductance is expressed in the unit reciprocal ohm or siemens.
Specific conductance :- Conductivity of the solution by all the ions produced from one gm equivalent is known as equivalent conductance.
A = Kv ( v is ml of solution containing one gm equivalent of electrolyte )
If ‘C ’ is the concentration of solution as gm per liter ( normality ) the volume v of solution in ml containing 1 gm – equivalent will be 1000/c Therefore, A = kv = V= 1000/c Unit of a is ohm- cm
Molar conductance ( u ) :- Conductance of solution by solution containing one gm- mole of electrolyte is known as molar conductance and it is denoted by u U = Kv = M= concentration of solution in moles / liter unit of U is ohm - cm² per mole
Cell constant :- K= If two electrodes ‘l’ cm apart and having area areas designed in conductivity cell and it is known as cell standard its unit is per cm or per meter. K = = conductance * cell constant
Resistance of 0.1 N Kcl solution in a conductivity cell was 702 ohms and was 0.148
K = Therefore, Cell constant = k*R = 0.00148 * 702 = 0.989 cm – = 1.03 cm-
Definition ( ˄0 ):- The maximum equivalent conductance at infinite dilution is known as equivalent conductance at infinite dilution and it is denoted by ˄0
Measurement of conductance :- Measurement of electrolyte conductance of solution for determining resistance of solution because conductance is the reciprocal of the resistance , generally Wheatstone bridge method is used to measure resistance of a solution
CONDUCTOMETRIC TITRATION :-
Strong Acid versus strong base titration :- e.g. :- strong acid = hcl taken in conductivity cell strong base = NaOH taken in burette when base is added H+ ions in solution are replaced by Na+
H+ ion of higher mobility will be replaced by slower moving Na+ . so, conductivity goes on decreasing until end point. After end point there will be increase in conductance due to net addition of Na+ + OH- in the mixture end point corresponds to the minimum conductance .
Y-AXIS-Sp. CONDUCTANCE From end point equivalence point from group normality of NaOH , the amount of Hcl can be calculated. Curve AB represents variation of conductance of mixture of progressively decreasing Hcl and increasing NaCl . portion BC indicates the conductance of NaCl formed on neutralization of Hcl , along with excess base added . At B there are neither aH+ nor OH- in excess and it is the equivalence point of titration.
Weak Acid versus strong base :- e.g. weak acid – CH3COOH ( acetic acid ) strong acid – NaOH
starting conductance of acetic acid is low and it further decreases due to depression in its dissociation by the common ion formed during early stage of neutralization.
After that the conductance increases slowly due to salt formed up to eq. point after that conductance increase faster
X-AXIS-mL OF BASE Y-AXIS-Sp. CONDUCTANCE K IS Eq. POINT Due to excesses of Na+ and OH- ions added.
1ml 1N NaOH = 60Mg of acetic acid from end point or eq. point volume , normality of NaOH we can calculate amount of acetic acid in solution.
Weak base against strong acid :- e.g. :- strong acid – Hcl ( from burette ) weak base – NH4OH ( conductivity cell )
initially there is low conductance by weak electrolyte but during titration there is formation of strong electrolyte NH4Cl there for conductance gores on increasing up to equivalence point. After eq.point the conductance increase very rapidly because of fast conducting H+ cl- added remains unreacted in the titration mixture.
Thus, the slopes of lines before and after equivalence being difference
X-AXIS-mL OF BASE Y-AXIS- CONDUCTANCE K IS Eq. POINT
From equivalence point noted from a graph normally of hcl . we can calculate amount of base titrated.
Precipitation titration :- Precipitation titration can be carried out by conductivity measurements.
e.g. – Kcl versus AgNO3 is added from burette and conductance of Kcl solution observed at various points .
The conductance of Kcl decrease slowly up to equivalence point because greater mobility of cl – are replaced by lower mobility NO3 – ions. Because conductance difference in them is not large therefore conductance decreases slowly up to equivalence point. After that conductance increases rapidly due to addition of Ag + and NO3 – ions from burette.
X-AXIS-mL OF BASE Y-AXIS- CONDUCTANCE E IS Eq. POINT
|
References:-
1. Engineering Chemistry by O.G. Palanna, Tata Magraw Hill Education Pvt. Ltd.
2. Textbook of Engineering Chemistry by Dr. S. S. Dara, Dr. S. S. Umare, S. Chand & Company Ltd.
3. Textbook of Engineering Chemistry by Dr. Sunita Rattan, S. K. Kataria& Sons Publisher