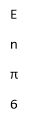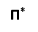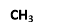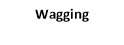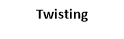UNIT 5
SPECTROSCOPIC TECHNIQUES
[A]uv – visible spectroscopy
- Ultraviolet – visible spectroscopy
Spectroscopy: -
- Spectroscopy is the branch of science that deals with the study of interaction of electromagnetic radiations with matter.
- The spectroscopic techniques make use of either absorbed or emitted or scattered electromagnetic radiation by atoms or molecules present in the substance which help in qualitative and quantitative analysis of substances.
Electromagnetic radiations: -
- Electromagnetic radiations are a form of energy that is transmitted through space with high velocity.
- Electromagnetic radiations consist of discrete packets of energy which are called photons.
- A photon consists of an oscillating electric field (E) and an oscillating magnetic field (M)
Terms: -
- Wavelength (X): -
Wavelength of radiation is the distance between two crests or peaks of either electric or magnetic component. it is expressed in meters.
2. Frequency (v): -
It is the number of waves that pass through a fixed point in unit time. it is expressed in reciprocal second (s-1) or hertz (hz).
3. Wave number (v): -
It is reciprocal of wavelength expressed as reciprocal centimetres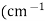 ).
).
The energy associated with electromagnetic radiations is given by,
E= hv = 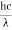
- E = energy of radiations
- H = planks constant
- V = frequency of radiation in hz
- C= velocity of light
Electromagnetic spectrum: -
- The electromagnetic spectrum consists of the arrangement of all types of electromagnetic radiations in the order of their increasing wavelength and decreasing frequencies.
Interaction of electromagnetic radiation with matter
The wavelength of radiation is as short as 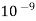 nm to as 1000km. the physical and chemical effects of the radiations are quite different due to different energies of photons.
nm to as 1000km. the physical and chemical effects of the radiations are quite different due to different energies of photons.
- e.g.: - absorption of a particular wavelength in the range 2.5 to 50 microns can cause vibration for certain covalent bond.
- When an electromagnetic radiation falls on matter, it interacts with atoms / molecules present in the matter, which exists in discrete energy states.
1) Types of energies: -
Like atoms,molecules possess a set of discrete energy levels.
Rotational energy: -
- This type of energy is associated with rotation of molecule about the axis passing through centre of gravity.
|
- Vibrational energy: -
- This type of energy is associated with vibrations of molecule such as stretching or bending of bonds.
|
ii. Electronic energy: -
- This type of energy is associated with motion of electrons from ground state to excited state.
- Relation between these energies can be shown as
E Elec>E vib > Erot
- Total internal energy of molecule is given by: -
Etot = Elec + Evib + Erot
2) Molecular energy levels: -
- Total energy states of molecule include electronic vibration and rotational levels.
Rotational energy levels: -
- Are very closely spaced little energy is required for rotational energy transitions which occur in microwave region.
|
Vibrational energy levels: -
- Are spaced further apart. vibrational energy transition required more energy these transitions occur infrared region of spectrum.
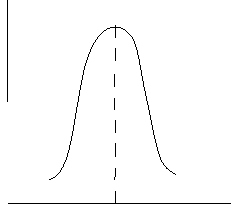
Absorption spectrum: -
- A plot of the energy absorbed as a function of wavelength or frequency is called absorption spectrum.
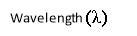 The wavelength at which there is maximum absorption is called as wavelength of maximum absorption (λ)
The wavelength at which there is maximum absorption is called as wavelength of maximum absorption (λ)
Uv – visible spectroscopy: -
- Uv and visible radiation are energetic electromagnetic radiations that bring about electronic excitations.
- The electromagnetic radiations with weakness range 10 – 400nm are called ultraviolet radiation and 400nm are called visible light radiations.
Lambert – beer laws of absorption
Lamberts law: -
|
- The rate of decrease in intensity of radiation is directly proportional to path length of solution when the monochromatic light passes through a solution of constant concentration.
- Mathematically the law can be written as,
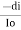 ά dxe
ά dxe
Therefore,
Io = intensity (radiantpower) of the incident radiation.
Dx = small thickness of solution or path length.
Di = small decrease in intensity of light = IO – It.
Intensity or radiant power is the number of photons per unit area per second
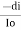 = KI.dxe
= KI.dxe
Integrating within limits
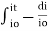 =
= 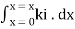
Therefore,(ln it – ln io) = ki. x
In io/it = ki.x
Lambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration.
Beer’s law: -
- The rate of decrease in intensity of light proportional to concentration of solution for a fixed thickness of solution.
Mathematically the low can be written as,
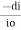 ά dc
ά dc
Integrating within limits
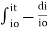 =
= 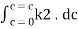
Therefore,
- -( ln it – ln io) = k2.c
- Or ln io/it = k2c
- it = io. e-k2.c
In other words, beers law can be stated as the intensity of transmitted light decrease exponentially with increase of concentration, for a fixed path length of solution.
Combined lambert – beers law
|
The combined lamberts – beers law will be
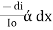 -- --------------------- (lamberts law for constant concentration)
-- --------------------- (lamberts law for constant concentration)
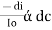 ----------------------- - - (beers law for constant thickness of solution)
----------------------- - - (beers law for constant thickness of solution)
Therefore,
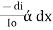 or
or 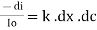
Integrating within limits
Ln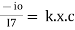
Or
Log 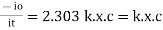
Therefore,
K is absorptivity constant
The term log 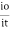 is defined as absorbane (a)
is defined as absorbane (a)
And transmittance (T) is defined as it/io
A= K.X.C Lambert – beers law
Therefore,
X is in om
C is in gm/lit
Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.
By taking unit concentration of sample in solution (c= 1 mole) and unit length of path (x = 1cm) the absorbance observed will be the value of the constant in lambert – beers equation.
A= E.X.C
Therefore,
E is known as molar absorptive or molar extinction coefficient.
- There is certain wavelength which is absorbed maximum and it is known as λ max. the wavelength is selected to analyse the sample by spectrophotometer.
- Absorption of UV radiation by organic molecule leading to different electronic transitions. Transition of electrons are of 4 types.
- 6-6*transition: -
- These transitions can occur in such compounds in which all the e- s are in involved in single bonds and there are no lone pair of any atom in the molecule.
- Maximum amount of energy is required for this type of translation.
- Absorption bond occurs in the ultraviolet region (100 – 135 nm)
2. N-π*transition: -
- These types of transition are shown by unsaturated molecule which also contain atom like oxygen,nitrogen, halogen etc...
- Minimum amount of energy is required for this type of transition
- Absorption bonds are also called R bonds which are very weak bonds observed in the wavelength region 270-320nm
3. N – 6*transition: -
- Saturated compounds with atom having lone pair of e° can undergo this transition.
- Absorption bonds occur in the near UV region 180-228nm.
4. ∏-∏ transition: -
- Compound having conjugated double like bonds like a butadiene etc. where absorption is very large and in the range of 210 – 28 nm. this bond is called K bond.
- Compounds containing only isolated double bond, where absorption is large and in the range of 160 – 175 nm is called E bond.
- B – bond are characteristics of aromatic and heteroaromatic molecule.absorption is board and in the range of 220 – 270 nm.
The order of energy required for electronic transition is,
|
1) CH2 = CH – CH2 – CH3: -
- Isolated ∏ bonds in molecule ∏ - ∏ electronic transition and absorption bond occur – 180 -220.
2) CH3 – CH2 – OH: -
- All sigma bonds with O atom bearing ion pair of electrons.
Hence molecule = n – 6 absorb maximum at about 200nm
3) CH3 – C – CH3: -
- There is a ∏ bond and O atom with the ∏ bonds bear with lone pair e°. hence the molecule will have n - ∏ transition – 270 – 350 nm. and another absorption for ∏ - ∏ transition at 180 – 200nm.
4) CH3 – CH 2 – CH2 – CH2 – CH3: -
- The molecule contains all sigma bonds with electronic transition 6-6 to absorb below 150nm.
Terms in UV- visible spectrophotometry
1) Chromophore: -
- A covalently unsaturated group responsible for electronic absorption in UV – visible region is called as the chromophore.
e.g.: - C=C, C=O, N=O etc.
2) Aero chrome: -
- A saturated group with lone pair of electrons which when attached to a chromophore changes both wavelength and intensity of absorption.
e.g.: - -NH2, -OH, -Cl etc groups.
|
3) Bathochromic: -
- The shift of absorption to a longer wavelength due to substitution or solvent effect is known as bathochromic shift or red shift.
4) Hypochromic shift / blue shift: -
- The shift of absorption to a shorter wavelength due to substitution or solvent effect is called as hypochromic shift or blue shift.
5) Hypochromic: -
- An increase in absorption intensity called as hyperchromic effect.
e.g.: -







6) Hypochromic effect: -
- A decrease in absorption intensity called as hypochromic effect.
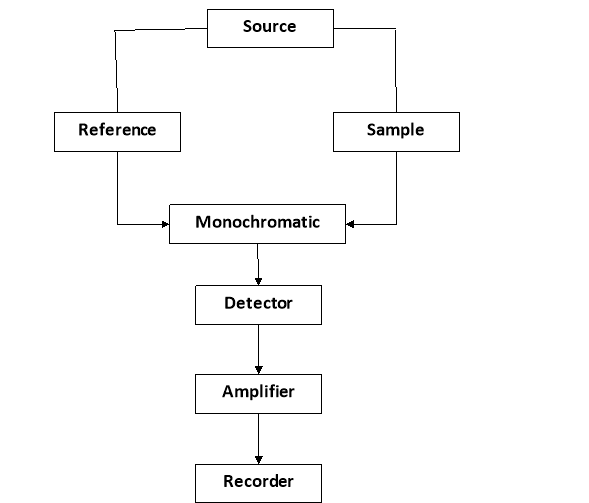
e.g.: - Instrumentation in UV – VISIBLE SPECTROPHOTOMETIC
(UV - visible spectrophotometer)
It consists of following parts: -
1) Source of radiation: -
- It should provide sufficient intensity over the wavelength region where absorption is to be measured for obtaining 300 – 1000 nm wavelength, an in candescent filament, lamp is used, hydrogen or discharge lamp is used.
2) Mono chromate: -
- A monochromator consists of entrance slit,collimator, a grating or prism, exist slit a good mono chromate provides very narrow wavelength bond.
- Glass prisms are used for work in the visible region, quartz prisms are used for UV region.
3) Gratings are of two types: -
2 types
a) Transmission grating: -
- It consists of a series of closely spaced parallel grooves on a transparent material a suitable grating for work in UV – visible region has 2500 -5000 lines per cm.
b) Reflection grating: -
- Are used more commonly it consists of closely spaced grooves on metallic film.
5. Slits: -
- The slits are used for selecting the desired wavelength from dispersed light by the monochromator slits are placed in the opposite sides of the prisms or grating.
6. Sample holder: -
- There is special glass tube is also called as cuvette or sample cell which does not absorb the light from uv – visible spectrum and the sample solution is kept in it
e. g: - quartz glass cuvettes are used in 200 – 300nm and fused glass cuvettes are used in 300 - 2500 nm.
7. Detector: -
- It is also called as transducers which convert electromagnetic radiation in an e- flow due to that photo current is developed which amplified by amplifier and recorded by recorder.
 Applications of uv – visible spectroscopy
Applications of uv – visible spectroscopy
- Qualitative analysis: -
- The technique is determining is used to determine presence or absence of chromophore.
2. Quantitative analysis: -
- UV –visible absorption spectroscopy can be used for quantitative determination of compound by using beer.
- Lamberts law = a= E*C
3. Detection of impurities: -
- UV – visible spectroscope is one of the best methods for determination of impurities in organic compound.
4. Study of kinetics of chemical reactions: -
- Kinetics study of chemical reaction is done by measuring change in concentration of reactant or product that show presence in UV – Visible spectrum.
5. Dissociation constant: -
- To find dissociation constant for weak acid and weak base uv – visible spectroscopy can be used.
6. Structural information: -
- For getting structural information (chromophore, urochrome groups) uv – visible spectroscopy can be used.
Infra – red spectroscopy (IRS)
- IR region (12500cm to 10 cm) 0.8 um to 1000 microns wavelength
- IR spectroscopy is a powerful analytical technique for getting structural information of organic molecules and also for quantitative analysis of organic molecules.
Principle: -
- When a molecule absorbs certain wavelength from IR region, the energy in the photons of the wavelength, is utilized for stretching the atoms in the covalent bond or bending the two covalent bonds on an atom.
Types / modes of vibrations
- For nonlinear molecule absorb certain wavelength from IR region, the energy in the photons of the wavelength is utilized for stretching the atom in the covalent bond or bending the two covalent bonds on an atom.
- For linear molecule has (3n – 5) vibrational degrees of freedom as only two degrees of freedom are required to describe.
Number of fundamental vibrations
A] stretching vibration
1. symmetric 2. Symmetries
B] bending vibration
1.scissoring
2. rocking
3. wagging
4. twisting
Two basic categories
Stretching vibrations: -
- These are characterized by change in inter – nuclear distance.
Stretching vibration of are two types
Symmetric stretching vibrations: -
- These involve the movement of the atom with respect to a particular atom in a molecule in the same direction.
Asymmetric stretching vibrations: -
- These involve the movement of the atom with respect to particular atom in a molecule in the same plane but not in the same direction.
|
2) bending vibrations: -
These are characterized by change in the angle between two covalent bonds due to change in the position of the atoms. Relative to the original band axis.
These vibrations are four types: -
2. Rocking vibrations: -
3. Wagging vibrations: -
4. Twisting vibrations: -
Calculate the vibrational degree of freedom in following molecules: -
INSTUMENTATION IN I.R SPECTROSCOPY
I.R spectroscopy consist of following parts
1) Source of radiation: - For I R radiations, one of the following sourcescan be used.
A) Nerst filament: - Consist of temperature of earth oxides such as zirconium oxide, etc.
B) Global: - It is a rod of silicon carbide.
C) Incandescent wire: - It is spiral wire of nichrome.
D) Mercury are: - High pressure mercury is which consists of quartz tube containing mercury vapour can be used.
All these heated up to 2000°c, they produce mid – IR radiation.
2) Monochromator: - Prisms or gratings are used as monochromators. they are used for selecting desired radiation frequencies. prisms are normally made of metal halides like Nacl, KBr.
3) Sample cells and sampling of substances I R spectra can be obtained for solid,liquid, or gases. material containing sample must be transparent to the IR radiation, so the material made of certain salts like Nacl,KBr, LiF etc. are used.
4) Detector: - In IR spectroscopy, m thermal detectors are used.
a) Thermocouple: - it is uses two wire of diff materials welded together. b) Bolometer: - pt / Ni is used to form bolometer it is a type resistance thermometer. c) Goal detector: - it is a sensitive gas thermometer containing Zenon.
5) Recorder: - Recorder directly give the IR spectrum of a compound.
A) Identification of functional group: - In IR spectroscopy, all functional groups absorb characteristic radiation within definite range.
B) Identification of structure: -
2. Study of kinetics of a chemical reaction: -
e.g.: -
3. Hydrogen bonding: -
e.g.: -
Aliphatic: - OH free 3650 cm-1 Aliphatic – OH hydrogen bonded 3350 cm -1.
4. Distinction between intermolecular and intra molecular hydrogen bonding: -
5. Detection in impurities: -
6. Determination of size of ring ketones: -
e.g.: -
|
References:-
1. Engineering Chemistry by O.G. Palanna, Tata Magraw Hill Education Pvt. Ltd.
2. Textbook of Engineering Chemistry by Dr. S. S. Dara, Dr. S. S. Umare, S. Chand & Company Ltd.
3. Textbook of Engineering Chemistry by Dr. Sunita Rattan, S. K. Kataria& Sons Publisher