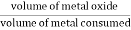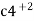UNIT- 6
CORROSION SCIENCE
Corrosion: -
- The destruction of metal by chemical or electrochemical attack of environment this process starting at the surface of metal known as corrosion.
2 types of corrosion on basis of corrodent / corrosion material
- Dry corrosion
- Wet corrosion
Dry corrosion: -
- Corrosion occurring due to direct attack of atmospheric gases like o2, co 2, so2, c12 moisture etc and by not flowing liquids is called as dry corrosion.
Rate of dry corrosion depends upon: -
- Temperature
- Moisture
- Chemical affinity between metal and gases
- Nature of oxide layer
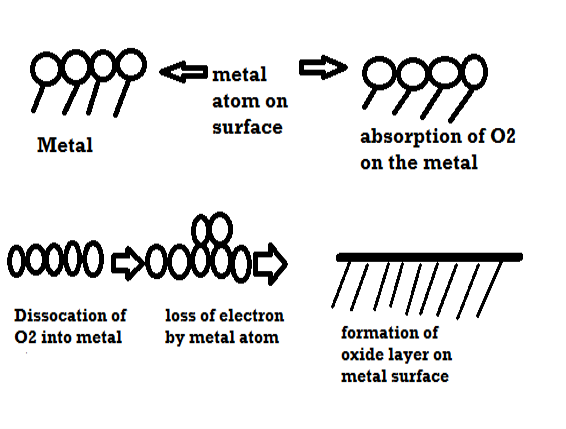
Mechanism of dry corrosion due to o2 gas there are 4 types: -
- Absorption of oxygen molecules on the metal surface
- Dissociation of oxygen atom into metal atom
- Loss of e- by metal atom
- Formation of oxide layer on the metal surface.
|
4 types of oxide layers: -
e.g.: - fe,Mg,Na,K,Ca, etc.
reaction: - 2fe + o2 – 2feO 2Mg + o2 – 2MgO
2. Non – porous oxide layer (protective): -
e.g.: - chromium,nickel, Sn (tin),lead,aluminium,zinc,bronze,various metalalloys .
reaction: - 1. 2AL + O2 – AL2O3
3. Unstable oxide layer: -
e.g: -Ag,AU, Pt.
4. Volatile oxide layer: -
dillingbedworth rule / ratio (PBR)
Then,
Oxide layer – porous Else, oxide layer – nonporous
PBR =
If, PBR > 1 but PBR < 1.45
Metal oxide layer – nonporous(protective)
B] Mechanism of dry corrosion due to other gases: -
a) Dry corrosion due to chlorine: - Reaction: - 1. 2Ag + Cl2 – 2AgCl
Agcl forms thin protective (non-porous) layer on the metal surface. Non corrosion taking place Sn + 2cl2 – SnCl4 SnCl4 forms thin, non-protective layer on the metal surface Corrosion taking place
b) Due to So2: -
Reaction: - SO2 + H2O – H2SO3 SO2 + H2O ½ O2 – H2SO4
c) Due to oxides of nitrogen: -
Reaction: - NO2 + O3 – NO3 + O2 NO 2 + NO3 – N2O5 N2O5 + H2O – 2HNO3
d) Decarburisation: -
e) H2 embrittlement: -
f) Presence of co2: -
H2O + co2 – H2CO3 Fe + CO2 – FeO + CO
EMF series and galvanic series
2) wet corrosion: -
2types: - A) Galvanic cell wet corrosion B) Conc. cell wet corrosion / differential wet corrosion
Galvanic cell wet corrosion: -
Conc. cell wet corrosion: -
Possibilities: -
Possibilities: -
|
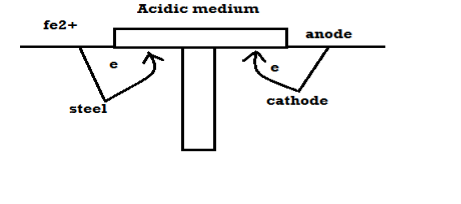
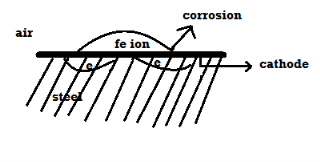
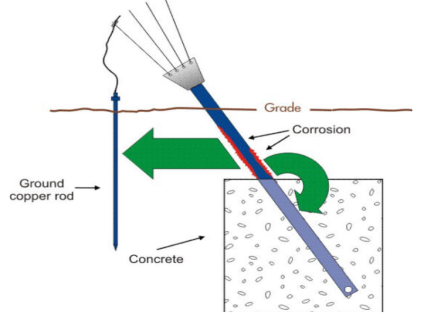
Mechanism of wet corrosion: -
M – m+n + ne-
2. Reaction on cathode: -
a) H2 liberation (H2 Evolution): -
b) O2 absorption: -
C) Plating: -
Factors influencing rate of corrosion. A] Nature of metal B] nature of environment
Nature of metal: -
Position of metal in galvanic series: -
Relative areas of anode and cathode: -
Purity of metal: -
Physical state: -
e.g: - steel get corroded fast than the cost iron because grain size of steel < grain size of cast ion.
Nature of oxide film: -
Then corrosion take place
Then no corrosion takes place
Over – vtg (over – potential): -
e.g: -Zn,Pb,Cr,Ni, etc.
B] nature of environment: - 1) temperature: -
High tempo, according to nearest equation electrode potential is high.
2) moisture: -
Higher is the rate of dry and wet corrosion
3) PH: -
4) conductivity of medium: -
5) nature of ions: -
Pour baix diagrams Pour baix diagrams are the plots of: - A] potentials of metal VS PH of media B] rate of corrosion vs voltage applied
Merits: -
Demerits: -
|
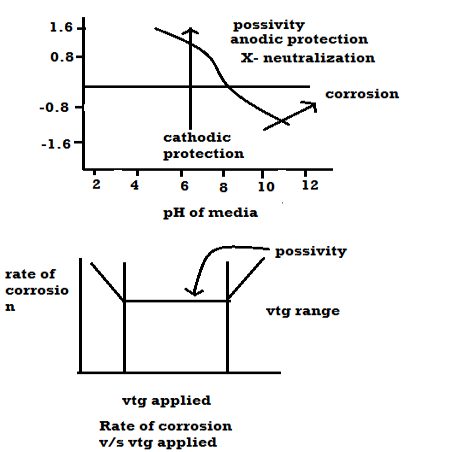
One way to minimize the dissimilar metal interaction that causes the corrosion of the structural steel anchor is to break the electrical path between these two components by adding insulators.
|
|
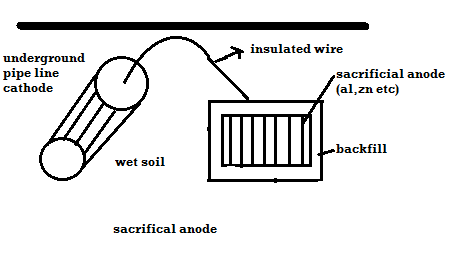
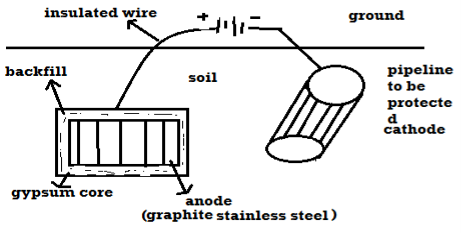
Principle: - The metal to be protected is forced to behave as cathode. 2 types: -
A) By using sacrificial anode Methods / processes: -
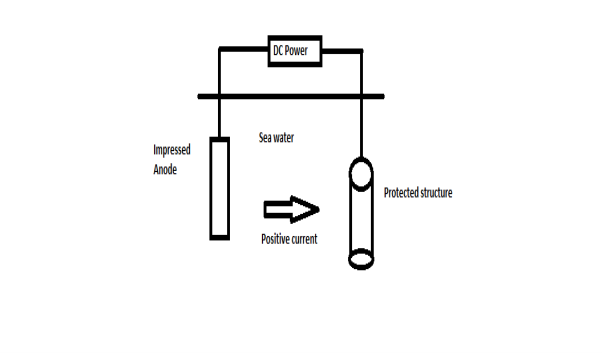
B) By using impressed current: - Method: -
Limitations: -
B] anodic protection: - Principle: - A metal or alloy having wider range of passivity vtg, is made anode and the vtg is passivity range is applied over it do control its corrosion even by strong corroding media.
Application: -
Advantages: -
Limitations: -
It is applicable for only those metals which show active – passive behaviour.
Metallic coatings and its types Metallic coating: - Cleaning methods: - 2 types: -
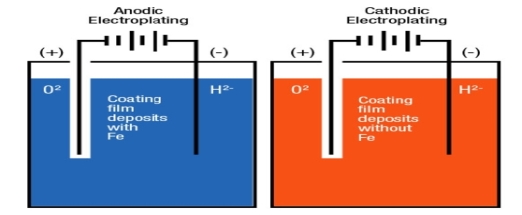
Anodic coating: - Coating metal is higher placed than the base metal then it is called as anodic coatings. e.g.: -galvanizing cathodic coatings: - if coating metal is lower placed in galvanic series than base metal then it is called as tinning. Methods of applying metallic coatings: -
Hot dippingprocesses: -
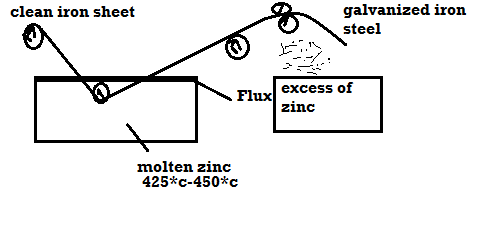
1) Galvanizing: - Method: -
Applications: -
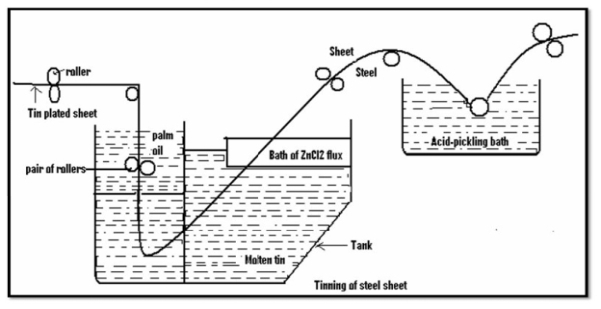
Tinning: - Method: -
Applications: -
2. Food material can be stored in tin containers
3. CV wire before involution by rubber are tinned.
4. Tinned or brass vessels or sheets are used for making cooking vensiles and parts of reflectors.
Principle: - Electroplating is a method in which coating metal is coated on the base metal on the basis ofelectrolysis principle.
Processes: -
Metal ions in a solution migrate towards the article and get deposit on the base metal in the form of coating layer.
At anode (coatingmetal)
Advantages: -
Application: -
In this method the base metal articles are packed in the powder of coating metal and heated to temp. just below the M.P of lower melting component of them. It is useful for making alloys of metals.
There are 3 types of cementation
Application: -
Metal cladding: - Metal cladding is a process in which a thin sheet of coating metal is bonded to the base metal on one or both the sides.
Clads: - coating metal sheets
Clad metals; -pb,Ni,Cu,Ag,Cr, al etc. and alloys like stainless steel,brass, nickel alloy, lead alloy.
Base metal: -brass, mild steel.
Method: -
Applications: -
|
References:-
1. Engineering Chemistry by O.G. Palanna, Tata Magraw Hill Education Pvt. Ltd.
2. Textbook of Engineering Chemistry by Dr. S. S. Dara, Dr. S. S. Umare, S. Chand & Company Ltd.
3. Textbook of Engineering Chemistry by Dr. Sunita Rattan, S. K. Kataria& Sons Publisher