GROUP-B
SYSTEMATIC CHEMISTRY OF THE ELEMENTS
Position in P.T
To attain the nearest noble gas configuration, both the halogens and the hydrogen need one electron to complete their octet and duplet, therefore hydrogen can gain one electron to produce a uni-negative ion. Hydrogen has an electrogene configuration of 1 and that of Fluorine is 2,7, both of them gain one electron each to attain the noble gas configuration of helium and neon, respectively. Like halogens, it forms a diatomic molecule and several covalent compounds.
2Na + Cl2 → 2NaCl
H2 + Cl2 → 2HCl
Similarities to Halogens
Noble Gas Configuration: Halogens have seven electrons in their last shell and can gain one electron to gain its noble gas configuration, similarly hydrogen gains one electron to complete its valence shell having a configuration of 1.
Electronegativity: They also share the same electronegative nature. Hydrogen also gains one electron (not looses) to become stable and so do halogens.
H + e‾ → H‾
Cl + e‾ → Cl‾
Diatomic Molecules: Both hydrogen and halogens form diatomic molecules. Hydrogen forms H2 and, halogens are Cl2, F2 etc
Reaction with Metals: Metallic hydrides are formed, when hydrogen reacts with metals, in the same way halogens also react with metals to form metal halides
2Na + H2 → 2NaH
Ca + H2 → CaH2
Covalent Bonding: Covalent bonding with molecules when both the halogen and Hydrogen combine with non-metals.
Differences with Halogens
Structure of Atom: Hydrogen has only one electron in its outer shell. All halogens have seven electrons in their last shell
Size of Atom: The size of the H- ion is much larger than those of the ions of Halogens. This is because hydrogen has only one electron and proton and the pull of the nucleus is less.
Reaction with Water: Also, unlike halogens, the hydrogen ion H- is unstable in water.
Isotopes of Hydrogen
Properties of Isotopes of Hydrogen
The three naturally occurring hydrogen isotopes are:
1H (protium), 2H (deuterium), and 3H (tritium). The other highly unstable ones are (4H to 7H) are processed in the lab, and do not occur naturally.
Of the naturally occurring isotopes, the most stable hydrogen radio isotope is tritium with a half-life of 12.32 years. The other heavier isotopes have a half-life less than a zeptosecond (10-21 sec). Of these, 5H is the most stable, and the least stable isotope is 7H.
Protium
H is the very common hydrogen isotope and found in abundance in nature with almost more than 99.98%. The nucleus of the protium contains of only a single proton (atomic number = mass number = 1) and neutrons are absent in the nucleus. And its mass is 1.007825 amu. Hydrogen generally occurrence is diatomic in the form of hydrogen gas H2, hydrogen also combines with other atoms in the compounds. The occurrence of monoatomic hydrogen is rare. The H–H bond is one of the strongest bonds in nature, with a bond dissociation enthalpy of 435.88 kJ/mol at 298 K. therefore, H2 dissociates to only a minor extent until higher temperatures are reached. At 3000K, the degree of dissociation is only 7.85%. Hydrogen atoms are so reactive that they combine with almost all elements.
Deuterium
The other stable isotope of hydrogen is 2H, or deuterium (D), the natural abundance is around 156.25ppm in the oceans and around 0.0156% of hydrogen present on earth. The nucleus of deuterium is called as deuteron, consisting of one neutron and one proton (mass number = 2), due to the presence of neutron in the nucleus deuterium is twice the mass of protium (deuterium has a mass of 2.014102 amu, compared to the mean hydrogen atomic mass of 1.007947 amu). Deuterium occurs in trace amounts naturally as deuterium gas, written 2H2 or D2, but is most commonly found in the universe bonded with a protium 1H atom, forming a gas called hydrogen deuteride (HD or 1H2H).
When chemical aspects are noticed deuterium performs similar to protium, but they differ in bond energy and length of compounds of heavy hydrogen isotopes that are bigger than the isotopic differences than any other element. The bonds of deuterium and tritium are much stronger than protium, some of these differences are significant in biological reactions. Deuterium replaces the normal hydrogen in water molecules to form (D2O), which is about 10.6% denser than normal water. Heavy water is slightly toxic in eukaryotic animals, with 25% substitution of the body water causing cell division problems and sterility, and 50% substitution causing death by cytotoxic syndrome (bone marrow failure and gastrointestinal lining failure).
Is in NMR, as it requires compounds of interest to be dissolved in solution, the solution signal should not register in the analysis. As NMR analyses the nuclear spins of hydrogen atoms, the different nuclear spin property of deuterium is not ‘seen’ by the NMR instrument, making deuterated solvents highly desirable due to the lack of solvent-signal interference.
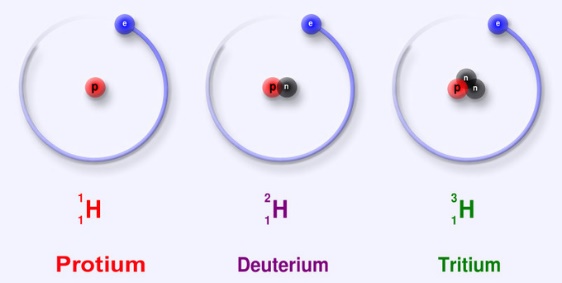
Fig :1Isotopes of Hydrogen The three naturally occurring isotopes of hydrogen.
Tritium
3H is known as tritium and contains one proton and two neutrons in its nucleus (mass number = 3). they are radioactive in nature that decay into helium-3 through beta-decay followed by the release of 18.6 keV of energy. It has a half-life of 12.32 years. Tritium occurs rarely on earth; trace amounts may be found when the cosmic rays interact with the atmosphere.
Heavier Synthetic Isotopes
4H has in its nucleus one proton and three neutrons, they are highly unstable isotope of hydrogen. Tritium is bombarded with fast-moving deuterium nuclei in the lab to produce this molecule. In this experiment, the tritium nuclei captured neutrons from the fast-moving deuterium nucleus. The presence of the hydrogen-4 was deduced by detecting the emitted protons. Its atomic mass is 4.02781 ± 0.00011 amu. It decays through neutron emission with a half-life of 1.39 ×10−22 seconds.
5H consists of four neutrons and a proton, this is also a highly unstable heavy isotope of hydrogen, they are synthesised in the lab by bombarding tritium with fast moving tritium nuclei. One tritium nucleus captures two neutrons from the other, becoming a nucleus with one proton and four neutrons. The remaining proton may be detected and the existence of hydrogen-5 deduced. It decays through double neutron emission and has a half-life of at least 9.1 × 10−22 seconds.
6H decays through triple neutron emission and has a half-life of 2.90×10−22 seconds. It consists of one proton and five neutrons.
7H has six neutrons and one proton, it was first synthesised by the Russian group in 2003 by bombarding hydrogen with helium-8 atoms that are donated to the hydrogen’s nucleus. The two remaining protons were detected by the “RIKEN telescope”, a device composed of several layers of sensors, positioned behind the target of the RI Beam cyclotron.
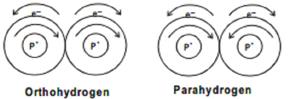
Fig 2: the Spins that occur in para and Ortho Hydrogen
The nucleus of the hydrogen atom spins about an axis like a top. When two hydrogen atoms combine, they form molecular hydrogen. Thus, depending on the direction of the two protons in the nucleus the following two types of hydrogen molecules are known.
When two hydrogens combine, molecular oxygen is formed, the nucleus of the hydrogen spin about their axis like a top.
Therefore, depending on the direction of the protos present in the nucleus. Two types of hydrogen are known to be formed. The two protons of the nuclei of both H-atoms, when they spin in the same direction, they are called as Ortho hydrogen, and when the protons of the nuclei of both H-atoms spin in opposite direction, it is called as parahydrogen.
The ordinary hydrogen contains 75% ortho and 25% para hydrogen at room temperature, but as temperature is lowered, the equilibrium shifts in favour of para form. At 25K There is 99% para and 1% ortho hydrogen. The change in the proportion of the two forms of hydrogen requires a catalyst such as platinum or atomic hydrogen or silent electric discharge.
The para hydrogen form was prepared originally made by absorbing ordinary hydrogen in activated charcoal present in a quartz vessel kept at a temperature of 20k. The charcoal absorbs almost pure para hydrogen. By this method, pure para hydrogen can be isolated.
Conversion of para into ortho hydrogen
Ortho hydrogen is more stable than para hydrogen. The para form is transformed into ortho form by the following methods.
1. By treatment with catalysts like platinum or iron
2. By passing an electric discharge
3. By heating to 800oC or more.
4. By mixing with paramagnetic molecules like O2, NO, NO2.
5. By mixing with nascent hydrogen or atomic hydrogen.
Properties: Ortho and para hydrogen are similar in chemical properties but differ in some of the physical properties.
(i) Melting point of para hydrogen is 13.83K while that of ordinary hydrogen is 13.95 K.
(ii) Boiling point of para hydrogen 20.26K while that of ordinary hydrogen is 20.39K.
(iii) Compared to the ordinary liquid hydrogen, the vapour pressure of liquid para hydrogen.
(iv) the magnetic moment of para hydrogen is twice than that of proton, where as that of para hydrogen is zero since the spins neutralise with each other
(v) Internal molecular energy is lower in parahydrogen than the ortho form.
Hydrides: ionic, covalent, metallic and intermediate.
Hydride, are anions of hydrogen, where the hydrogen atoms show basic, nucleophilic or reducing properties. Basically, in a hydride the hydrogen has the oxidation number equal to −1. Some of the most popular examples include water (H2O), methane (CH4) and ammonia (NH3).
When hydrogen reacts with any other element except noble gases they are called as hydrides, they are compounds of hydrogen with less electromagnetic elements. Hydride gap is observed in the periodic table, hydride formation is not visible from VA group elements. However, the properties may vary depending on the type of intermolecular force that exists between the elements, its molecular masses, temperature, and other factors.
Hydrides are mainly divided into three major types or groups. The categories are decided based on what elements the hydrogen forms bonds with or simply on the basis of chemical binding. The three types of hydrides are ionic, covalent, and metallic hydrides.
Ionic or Saline Hydrides
Example of Ionic Hydrides: Nah, KH, CaH2, etc. These contain hydrogen as the negatively charged (H–) ion.
Covalent hydrides are formed when hydrogen reacts with other similar electronegative elements like Si, C, etc. The most common examples are CH4 and NH3. In general, compounds that are formed when hydrogen is reacted with non-metals are called covalent hydrides. The compound shares a covalent bond and are either volatile or non-volatile compounds. Covalent hydrides are also either liquids or gases.
Example of Covalent Hydrides: SiH4 (silane)
Metallic Hydrides
When a hydrogen compound forms a bond with any other metal they are known as metal hydride. the bond formed is mostly covalent but, in few cases, hydrides form ionic binds, these are generally formed transition metals and show properties like high melting point, boiling point, hard and non-stoichiometric in natures.
Example of Metallic Hydrides: TiH aluminium, cadmium, magnesium, etc.
Metal hydrides are also known as interstitial hydrides. When hydrogen molecule reacts with the d- and F-block elements the interstitial hydrides are formed. Metals of group 7, 8, and 9 do not form hydrides. They do conduct heat and electricity but not to the extent of their parent metals.
Borderline hydrides: they are the hydrides formed from hydrogen and the elements of the periodic table present in group11 and group 12 and indium (In) and thallium (TI). they show properties that are intermediate between ionic hydrides and covalent hydrides. Hydrides are chemical compounds consisting of a hydrogen and a metal that acts as a negative ion.
Borderline hydrides/ Intermediate hydrides display bonding properties between covalent and ionic bonds, a common example is CuH copper hydride, is a reducing agent that looks like a spongy- reddish-brown substance. At room temperature they catalyse the oxidation of hypo phosphorus to phosphorous acid and liberates hydrogen gas when subjected to heat. ZnH2 is also a solid at room temperature that breaks down at 90 °C, but even left alone decomposes over several days to zinc metal and hydrogen gas. Hydrogen telluride (H2Te) and hydrogen selenide (H2Se) are both borderline hydrides of high volatility that produce strong, unpleasant odours.
Hydrogen Ion
The isolated hydrogen (H+ Ion) can only exist in a nearly particle-free space (high vacuum) and in the gaseous state siince the bare nucleus can easily combine with other particles (electrons, atoms, and molecules). The name hydrogen ion is commonly used to refer to the hydrogen ion found in water solutions, where it occurs as the combined molecule H+, H2O.
Hydrogen ion formula in Aqueous solution
The hydronium or oxonium ion is represented by the formula H+H2O, which is also written as H2O, which is also written as H3O+.The amount of hydrogen ion in a water solution is used to determine a substance’s acidity; the higher the hydrogen ion concentration, the more acidic the solution and the lower the pH.
Hydrogen Ion Concentration
Acidity or alkalinity is determined by the hydrogen ion concentration.
Let’s take the case of water. H2O is the formula for water, as you already know. The majority of water molecules are in a highly stable shape known as H2O. A small percentage of such compounds, however, have broken down into hydrogen ions (H+) and hydroxide ions (OH-).
In fact, the pH of water is determined by the balance of hydrogen and hydroxide ions.
The solution is acidic when the hydrogen ions outnumber the hydroxide ions. If the situation is reversed, the solution is alkaline. For any solution, the following relationship between the densities of hydrogen ions (H+) and hydroxide ions
(OH-) is observed if the temperatures do not change: (H+) (OH-) is observed if the temperature does not change: (H+) (OH-)=Kw=10-14(=fixed) at 25ºC.
(Kw is called the ion product of water).
In pure water or neutral solution:
[H+] = [ OH- ]
[H+] = [ OH- ] = √ (Kw) = √ 10 -14 = 10 -7
If the value of either [H+] or [ OH-] is known the value of the other can be determined.
Thus, pH is determined by hydrogen concentration.
pH = - log 10 [H+]
Uses of hydrogen ion
In photosynthesis, hydrogen ions drive ATP synthase. As the hydrogen ions are forced through the membrane, a high concentration occurs within the thylakoid membrane and a low concentration occurs in the cytoplasm, Osmosis on the hand causes H+ ions to force its way out of the membrane through ATP synthase. The protons will spin the ATP synthase which produce ATP using Kinetic energy to escape. This occurs in cellular respiration as well, though the concentrated membrane is the inner membrane of the mitochondria rather than the plasma membrane.
The acidic or basic essence of a compound is often determined by the concentration of hydrogen ions which is calculated as pH, H+ and hydroxide anions are formed when water molecules break known as water self-ionization.
H2O2: Preparation, Properties, Structure and Uses.
It is often referred to as water with one more oxygen atom. It is acidic in nature and PH is about 4.5. It is 100 percent degradable compound.
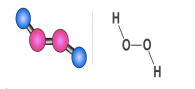
Fig 3: Structure of hydrogen peroxide
Structure of Hydrogen Peroxide
The structure of hydrogen peroxide is non-planar. H2O2 has an open book structure with O – O spins. The dihedral angle is 111°. The O-Obond length is 145.8 pm and the O-H bond length is 98.8 pm (which is equal to 9.88 × 10-13 m). The following diagram will clearly show what an open book structure means.
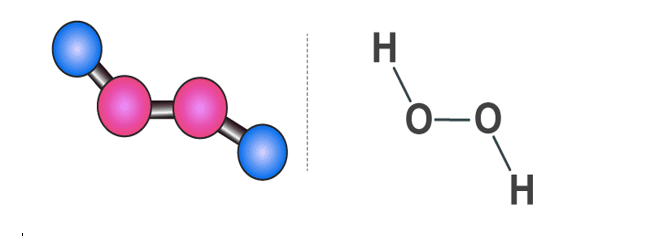
Fig 4: Hydrogen Peroxide Structure
There are two planes in this structure and each plane has one O-H bond pair, the angle between both the planes is 90.2°.
Preparation of Hydrogen Peroxide
Laboratory Methods of Preparation
BaO2.8H2O(s) + H2SO4(aq) → BaSO4(s) + H2O2(aq) + 8H2O(l)
Industrial Method of Preparation
Hydrogen peroxide is prepared by the electrolysis of 30% Ice -cold H2SO4. When acidified sulphate solution is electrolyzed at high current density, peroxodisulphate is obtained. Peroxodisulphate is then hydrolysed to get hydrogen peroxide.
2HSO–4(aq) [Electrolysis] → HO3SOOSO3H(aq) [Hydrolysis] → 2HSO–4(aq)+2H+(aq) +H2O2(aq)
Reaction Mechanism
Electrolyte: 30% dilute H2SO4
Cathode: Pb wire
Anode: Pt rod
2H2SO4 → 2H+ + 2HSO–4
At Cathode: 2H+ + 2e– → H2
At Anode:
2HSO–4 → H2S2O8 + 2e– ⇒ Peroxide Sulphuric Acid [Marshall’s acid]
H2S2O8 + H2O → H2SO5 + H2SO4 ⇒ Peroxomono Sulphuric Acid [Caro’s acid]
H2SO5 + H2O → H2SO4 + H2O2
Commercial Methods of Preparation
Auto-Oxidation Method:
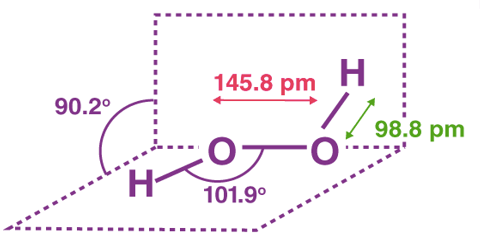
Fig 5:Auto-Oxidation Method of Preparation of H2O2
Properties of Hydrogen Peroxide
Physical Properties:
Chemical Properties:
Reaction: Pbs + 4H2O2 → PbSO4 + 4H2O
Oxidises KI to Iodine.
Reaction: 2KI + H2O2 → 2KOH + I2
Oxidizes nitrites to nitrates.
Reaction: NaNO2 + H2O2 → NaNO3 + H2O
Oxidizes acidified Potassium ferrocyanide.
Reaction: 2K4Fe (CN)6 + H2SO4 + H2O2 → 2K3Fe (CN)6 + K2SO4 + 2H2O
Oxidizes sulphites to sulphates.
Reaction: Na2SO3 + H2O2 → Na2SO4 + H2O
Oxidizes CrO-24 in an acid medium to chromium Peroxide CrO5.
Reaction: CrO-24 + 2H+ + 2H2O2 → CrO5 + 3H2O
Uses
(a) Idea of Mohs’s scale of hardness of minerals
The hardness of Gemstones and minerals are ranked by the common methods of Mohs’s (Mohs) scale. In 1812 a German mineralogist Friedrich Mohr devised this scale grades minerals on a scale from 1 (very soft) to 10 (very hard). As the Mohs scale is a relative one, it shows the difference between the hardness of a diamond and that of a ruby is much greater than the difference in hardness between calcite and gypsum. As an example, when diamond (10) is compared to Corundum (9) it is about 4-5 times harder, and 2 times harder than The Mohs rating may slightly differ depending on the individual sample the values will be ear same value. Half-numbers are used for in-between hardness ratings.
How to Use the Mohs Scale
A mineral with a given hardness rating will scratch other minerals of the same hardness and all samples with lower hardness ratings. As an example, if you can scratch a sample with a fingernail, you know its hardness is less than 2.5. If you can scratch a sample with a steel file, but not with a fingernail, you know its hardness is between 2.5 and 7.5.
Gems are examples of minerals Gold, silver, and platinum are all relatively soft, with Mohs ratings between 2.5-4. Since gems can scratch each other and their settings, each piece of gemstone jewellery should be wrapped separately in silk or paper. Also, be wary of commercial cleaners, as they may contain abrasives that could damage jewellery.
Mohs Scale of Hardness
Hardness | Example |
10 | Diamond |
9 | corundum (ruby, sapphire) |
8 | beryl (emerald, aquamarine) |
7.5 | Garnet |
6.5-7.5 | steel file |
7.0 | quartz (amethyst, citrine, agate) |
6 | feldspar (spectrolite) |
5.5-6.5 | most glass |
5 | Apatite |
4 | Fluorite |
3 | calcite, a penny |
2.5 | Fingernail |
2 | Gypsum |
1 | Talc |
Mohs Scale History
While the modern Mohs scale was described by Friedrich Mohs, the scratch test has been in use for at least two thousand years. Aristotle's successor, Theophrastus, described the test around 300 BC in his treatise On Stones. Pliny the Elder outlined a similar test in Naturalis Historia, circa AD 77.
Other Hardness Scales
The Mohs scale is only one of a number of scales used to assess mineral hardness. Others include the Vickers scale, Brinell scale, Rockwell scale, Meyer hardness test, and Knoop hardness test. While the Mohs test gauges hardness based on a scratch test, the Brinell and Vickers scales are based on how easily a material can be dented. The Brinell and Vickers scales are especially useful when comparing the hardness values of metals and their alloys.
Holme's classification of metals into five groups
It is important to note that almost all the elements in the periodic table are metals especially of the 118 elements 91 are metals, they are furthur classified into alkali metals, alkaline earth metals, transition metals, and basic metals.
The properties of metals
The elements in the periodic table are categorised into metals and non-metals. Some elements in the periodic table exhibit properties of both metals and non-metal properties, hence they are called metalloids.
The properties of a metal include:
Noble metals
These metals do not form compounds and are non-reactive, they are pure in nature, this non-reactive property makes them efficient for coins and jewellery, some of the examples include palladium, copper, silver, rhodium, and osmium.
Alkali metals
These metals have low melting points and are very reactive, these metals are named so because they react with water to form alkalis, they are soft and hence can be easily cut. Some examples are potassium, lithium and sodium.
Alkaline earth metals
These metals are found as compounds with other minerals they are much harder, reactive, showing high melting point, but show reactivity less than alkali metals. Due to their reactivity – they seldom appear in their pure form. This group includes calcium, magnesium, and barium.
Transition metals
These metals are hard, shiny easily moulded and strong, some are also noble metals, they have a range of industrial applications. Some examples include gold, silver and iron
Other metals
These metals are sometimes known as poor metals or post-transitional metals, they have low boiling points and are soft in nature, they are generally located between the metalloids and transition metals on the periodic table and have various uses. Aluminium is used when soldering, for example as well as to craft utensils whilst lead is used in batteries. Other examples include gallium and bismuth.
Metalloids
These elements show properties of both metals and non-metals for example, silicon is lustrous like a metal, but also quite brittle like a non-metal. There are seven metalloids found in the periodic table and can be found on the border between the metals and non-metals.
General methods of extraction their position in electrochemical series
Classification of metals on basis of extraction methods:
With respect to techniques by which these metals are being extracted, metals are divided into following main classes:
Class 1:
Class 1, this class includes mainly alkali metals that are highly electropositive in nature. The metals are extracted in fused or molten form and are extracted by hydrolyses method.
Class 2:
This is similar to class one, but includes mainly alkaline earth metals. The metals are electropositive, the metals are extracted through electrolysis in molten form.
Class 3:
Electrolysis and reduction in used for reactive metals with high valency.
Class 4:
Metals such as copper, nickel and cobalt are extracted by Roasting and reduction methods are used for extracting heavy metals
Class 5:
Amalgamation and cyanide process of extraction are used for less reactive metals. Common example is the silver.
Major Steps in extraction:
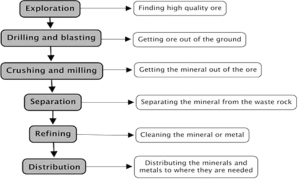
The extraction method includes several steps, that start from exploration of metal reservoirs to the final step of refining of metals. The main factors that are involved in this process are the physical properties of the metal and also the low cost of the process.
Exploration of ores:
The main techniques that are involved in exploration of high-quality ore deposits, in order to get the desired metals is as a follow:
Geological methods:
These methods include the basic collection and the presentation of geological data required to explore and find some ore. In some cases, these methods are combined with modern technologies to get desired level of quality ore. These include following
Geophysical methods:
These are types of analytical techniques which involve physical nature of ores. These methods are basically used for detecting ores which are difficult to be explored by chemical techniques. Main types of geophysical methods as follows:
Geochemical methods:
These types of explorations are done by chemically analysing rocks, sediments in lakes and soil of area to be explored. In this type of exploration methods, the sample is further analysed and refined in small scale at laboratory to get exact percentage of metal to be extracted and further detected. This exploration is of following main types:
Seismic methods involve estimation of the shapes and physical properties of Earth’s subsurface layers. This method is done by detecting the return of sound waves that are propagated through the Earth.
Hyperspectral technologies:
Unmanned ariel system to detect presence of ores is widely used method nowadays for ore detection. These methods area also known as remote sensing and they in combination with geophysical methods, have gained much importance in this industry.
Drilling and blasting:
Drilling and blasting are of foremost importance for further mining of ore. Methods to get ores out from reservoirs from drilling are as follows:
Down hole hammers
Mining:
Mining is basically an excavation produced in earth to extract minerals and ores. This industry has its history from very ancient times and together with engineering technologies, it has widely been used nowadays.
Surface mining:
In Surface mining, rocks and soil covering the ores and mineral deposits, are removed. It accounts to two third of world’s solid minerals. Surface mining is of following main types.
Underground mining:
It is used to extract ore from if it below the surface of the earth, economically and with as little waste as possible. Underground mining is of following two main types.
Crushing and milling
Once the metals are taken out from reservoirs they are subjected to further procedures of milling and crushing, in order to make it easy to process ore.
Separation from ore:
After the mining process, the metals need to be separated from the attached rocks, soil and some salts, this is performed by certain techniques, that depend on the type of compound and the metal to be extracted.
Gravity Separation: This method involves specific gravity of ore to be refined. Hydraulic washing/Gravity separation
It is also called “Gravity separation” or “Levigation”. In this method the ore is separated from the gangue by gravitational force. in the process the ore is first crushed into fine particles and made into a powder. The powdered ore is then passed through a water current. The process takes place on Wilfley table or
Hydraulic Classifier. This method mainly applies to the oxide and carbonate ores.
As the ore is more extensive it gets settled on the separatoe table while the gangue particles move along the water current stream.
Fig 6: Hydraulic washing
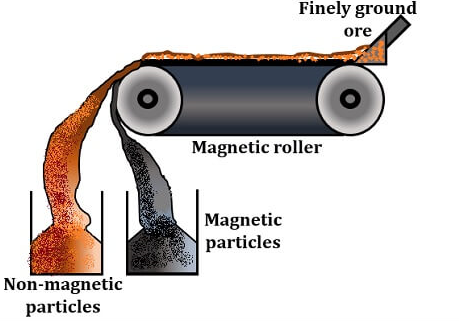
Magnetic separation
The magnetic property plays an very important role in this method. The ore is separated from the gangue particles by the magnetic properties of the ore or the matrix, the ore is crushed and the powder is run on a magnetic roller where one of the materials is magnetic and the other is non-magnetic.
The magnetic ore particles that are magnetic in nature will get attracted and attach to the magnetic roller, and the nonmagnetic gangue particles get repelled and fall in the heap from the conveyer belt.
Example: Fe (CrO2)2 (Chromite) is a magnetic ore, separated from the non-magnetic silicious gangue.
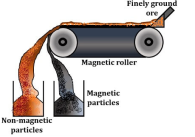
Fig 7: Magnetic separation
Froth floatation
In this process, the ore is finely ground and mixed in a little oil and made to pass into the bioreactor. The oil that is used in the froth floatation process is the pine oil. The bioreactor contains water onto which the mixture of ore plus oil is added through an inlet.
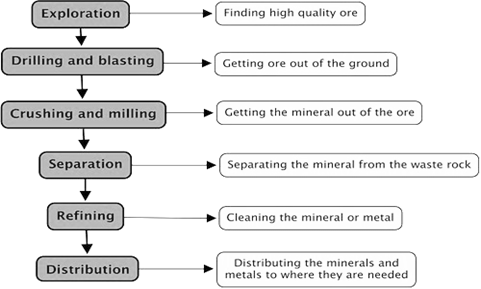
Fig : 8 Froth Floatation
Then, the mixture of ore, oil and water is thoroughly mixed or agitated by the rotating paddle (comprises impellers) that allows uniform mixing of all the components. There is constant airflow inside the medium, which leads to the formation of mineral froth (appears as a supernatant). Froth contains mineral particles that can be collected by transferring the mineral froth into the other bath, in which the ore free from gangue will settle down.
Liquation: This is used for ores having low melting point then impurities.
Extraction of crude metal from ore
Calcination
It is a process of heating the substance in limited oxygen supply, the thermal energy is utilised to change the chemical nature of the substance, and carbonates are usually converted into oxides by this method. The metal to be extracted is first converted into oxides to get its pure form by further procedure.
Roasting
In this method, elevated temperature is used to react a gas with metal on that temperature. Heat is constantly being given throughout the reaction. However, the higher temperature limit should not exceed boiling point of metal to be extracted.
Refining and purification of metal
Metals at the top of the series are good at giving away electrons. They are good reducing agents. The reducing ability of the metal increases as you go up the series.
Metal ions at the bottom of the series are good at picking up electrons. They are good oxidising agents. The oxidising ability of the metal ions increases as you go down the series.
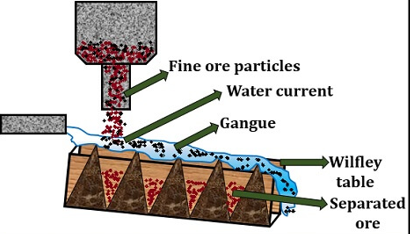
Fig : 9 Judging the oxidising or reducing ability from E° values
The more negative the E° value, the more the position of equilibrium lies to the left - the more readily the metal loses electrons. The more negative the value, the stronger reducing agent the metal is.
The more positive the E° value, the more the position of equilibrium lies to the right - the less readily the metal loses electrons, and the more readily its ions pick them up again. The more positive the value, the stronger oxidising agent the metal ion is.
Gibbs Free Energy
G = H - TS
where H, T, and S are the Enthalpy, temperature, and entropy. The SI unit for Gibb’s energy is the kilojoule.
Gibbs free energy combines both the enthalpy and entropy into a single value.
Gibbs free energy is the energy t with a that associates itself with a chemical reaction. It equals the enthalpy minus the temperature of the product and the entropy of the system.
G=H-TS
At constant temperature
ΔG = ΔH – TΔS
ΔG predicts the direction of a chemical reaction. If ΔG value is negative, then the corresponding reaction is spontaneous. If ΔG value is positive then reaction is non-spontaneous.
ΔGº=ΔHº-TΔSº
Where
ΔGº = Gibbs free energy (J or KJ)
ΔHº=enthalpy
T=Temperature
ΔSº=Entropy
Gibbs free energy is the energy that is available to do quality work.
(b) Principles of various concentration methods
The chemical methods include calcination, roasting and leaching for the concentration of ore.
Calcination is a very simple chemical process, where the solid material or the substance is heated in a controlled environment, in the process the temperature is also regulated. Calcination results a change in the substance both physically and chemically.
During calcination, the solid substances are subjected to heat at a very high temperature, this helps in removing volatile substances, water or oxidize the substance, hence the process is also called a purification process. The word Calcination has also been derived from the Latin word Calcinate which translates as “to burn lime”.
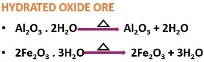
It is the chemical method of separating carbonate or hydrated oxide ores.
Calcination is usually carried out in furnaces, retorts, or kilns and often materials are racked over or stirred to make sure the product is uniform. One of the common arrangements that is used for calcination is the reverberatory furnace.
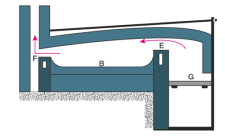
Fig 10: Calcination Process
The construction of the reverberatory furnace is different, however the in most cases the flames and hot gases from the fire come in direct contact with the material that is to be calcinated, but the fuel is separated from them.
In the above figure, the fire burns on the grate at G. Now the flames passing over the bridge at E are deflected downward by the low sloping roof of the furnace and pass directly over the surface of the charge or the material under calcination which is laid on the platform B. The fumes and hot gases then escape through the throat F into the chimney. The charge is spread out evenly on the bed as a thin layer.
A carbonate ore produces carbon dioxide under heat exposure.
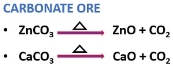
The hydrated oxide ore releases water under heat exposure.
Roasting
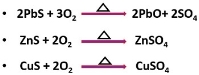
In roasting, the ore concentration or the ore is subjected to very hot air, this method is generally applied to sulphide minerals. The process proceeds under the influence of heat and air. During roasting, the sulphide ore is heated at a temperature below the sulphide melting point.
For example:
Leaching
It is a chemical method, in which the ore is treated with the suitable reagent to solubilize the ore.

The soluble ore or mineral can be separated from the insoluble gangue. After the separation of ore, it can further be recovered by several chemical methods.
Reduction of the Ore – by Pyrolysis
Pyrolysis, is a process that involves chemical decomposition of carbon-based organic materials with the use of heat, pyrolysis is also the initial step in gasification and combustion and occurs in the absence or near absence of oxygen, and hence is distinct from combustion, as it takes place sufficient presence of oxygen. The rate of pyrolysis increases with temperature. Pyrolysis is often carried out at 430 °C (about 800 °F) or higher in industries, whereas in smaller-scale operations the temperature may be much lower. Two well-known products created by pyrolysis are a form of charcoal called biochar, created by heating wood, and coke (which is used as an industrial fuel and a heat shield), created by heating coal. Pyrolysis also produces condensable liquids (or tar) and noncondensable gases.
Process
Pyrolysis helps to convert the organic material into its gaseous products, and a solid residue of carbon and ash and a liquid called pyrolytic oil (or bio-oil). there are two methods involved in pyrolysis for removal of contaminants from a substance are destruction and removal. In destruction method, the organic contaminants are broken down into compounds with lower molecular weight, whereas in the removal process, they are not destroyed but are separated from the contaminated material.
Organic materials that ‘crack’ or decompose in the presence of heat, to such materials pyrolysis is an important process. The examples include polychlorinated biphenyls (PCBs), dioxins, and polycyclic aromatic hydrocarbons (PAHs). Though pyrolysis is not utilised for destroying or removing inorganic matter such as metals, but pyrolysis can be used in techniques that render those materials inert.
Smelting, Role of carbon and other Reducing agents
After the preliminary treatment, the ore may be subjected to reduction process by one of the following methods depending upon its nature:
1. Smelting or Reduction with Carbon. In this method, the calcinated or roasted ore is treated with a known amount of coke or charcoal (reducing agent) and is subjected to high temperatures above its melting point, during the process an additional reagent is added to the ore to remove the impurities still present in the ore. This additional reagent is called as flux. Flux combines with the impurities to form a fusible product called slag.
Flux + Impurities → Slag
The flux is selected based on the nature of impurities, if the impurities are acidic in nature the flux added is basic in nature lime (Cao). Otherwise, if the impurities are basic in nature the flux added is acidic such as silica (SiO2) is used.
CaO + SiO2 → CaSiO3
(Basic (Acidic (Slag)
impurity) flux)
2. Reduction with Aluminium. Metal oxides like CR2O3 and Mn3O4 are not easily reduced with carbon. Aluminium is used as a reducing agent in such cases as they are more electronegative than chromium and manganese. The process of reduction of oxides with aluminium is called aluminothermy.
Some examples are:
Cr2 + 2A1 → Al2O3 + 2Cr
3Mn3O4 + 8Al → 4Al2O3 + 9Mn
The chemical methods are suitable for reduction of compounds of metals which are in the middle of the activity series.
3. Auto-reduction. Reducing agents are not required in few cases like certain metals that are obtained from their ores during roasting
For example, mercury is directly obtained by roasting its ore cinnabar (HgS) in air.
HgS + O2 → Hg(l) + SO2(g)
or
2Hgs(s) + 3O2(g) → 2HgO(s) + 2SO2g)
2HgO(s) + HgS(s) → 3Hg(l) + SO2(g)
4. Electrolytic Reduction. The highly electropositive elements such as alkali metals, alkaline earth metals and aluminium cannot be extracted by carbon reduction methods. They are extracted by the electrolysis of their fused salts. The process of extraction of metals by the use of electrolysis phenomenon is called electrometallurgy.
For example, sodium metal is extracted by electrolysis of molten sodium chloride containing other salts as impurities.
NaCI(l) → Na+ + Cl–
At cathode Na+ + e– → Na
At anode 2Cl‑ → Cl2 + 2e–
Hydrometallurgy
This is an extraction method involving metal or metal compounds from their ores, by pre-treatments that include the use of a leaching agent, separation of impurities and precipitation. It is used in the extraction of uranium, gold, zinc, silver and copper from low-grade ores. Hydrometallurgy has been improved by the development of process like solvent extraction and ion exchange.
Hydrometallurgy involves the recovery of the metals from a range of metal bearing sources. They involve a combination of extractive metals, sciences technology and chemistry for the recovery of metals from a wide variety of metal-bearing sources, that include ores, solutions, recycled materials, waste streams, intermediates and mineral concentrates that are converted into useful products for the society.
This wider field of technology is efficient in the on-site production of metals and forms an integral part of a growing number of metallurgical processes. It involves research, studies and novel technologies to obtain pure metals and better options for metal extraction. Hydrometallurgy revolves around three processes: leaching, metal recovery and solution purification.
The various techniques used in these processes include:
Electrolytic refining is a process that refines a metal especially copper by electrolysis process. During this process a large slab or chunk of the impure metal forms the anode and a thin strip of pure metal forms the cathode, the electrolyte which is a metal slat aqueous solution is used depending on the metal used.
The pure metal is formed at the cathode when an electric current that has sufficient voltage is applied by dissolving impure metal at the anode. Electrolytic refining is also sometimes referred to as Electrorefining.
The electrolytic refining of copper is the best example to understand the process clearly. Copper is obtained from its coal called as blister copper. The copper formed is about 98 to 99 per cent pure. However, the electrolytic process makes copper 99.95% pure, hence it can be used as a good product for electrical components.
A block of impure copper is taken as an anode or positive electrode. Copper sulfate which is acidified with sulphuric acid is used as a graphite-coated electrolyte along with pure copper tubes, as a cathode or negative electrode. In this phase of electrolysis copper sulfate divides into a positive ion of copper (Cu++) and a negative ion of sulfate (SO4—). The positive copper ion (Cu++) or cations travel towards the negative electrode made of pure copper where it absorbs the electrons from the cathode. Cu atom is deposited on the cathode’s graphite layer.
In the process of electrolytic metal processing, the cathode is coated with graphite, so that the concentrated material can be removed easily. This is the recent trend that is developed in the electrolysis procedures.
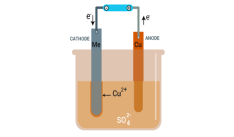
Fig 11: Electrolytic refining of copper
However, anode’s metallic impurities are also mixed with SO4, forming metallic sulphate in the electrolyte solution and dissolving. Impurities such as silver and gold that are not produced by a solution of sulphuric acid-copper sulphate settle down as the anode sludge or dust.
The deposited copper is then separated from the cathode and anode and is replaced by a new block of raw copper at a regular interval for electrolytic copper refining.
Chromatographic
This method is based on the principle that different components of a mixture are differently absorbed in an adsorbent.
The mixture is put in a liquid on a gaseous medium which is moved through the adsorbent, different components are adsorbed at different levels on the column
Later the adsorbed components are recovered using a suitable solvent.

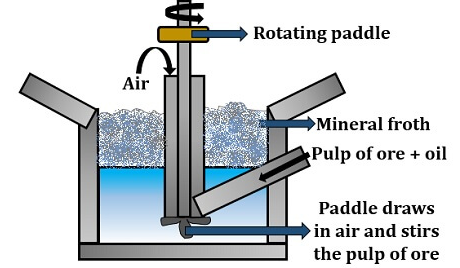
Fig 12: Separation of compounds using a stationary and a mobile phase
Ion exchange Solvent Extraction
Solvent extraction, also known as liquid-liquid extraction, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent.
Fig 13: Extraction of solvent
Importance of the process
The solvent extraction process was first developed as a tool of analytical chemistry. Every metallic element of the periodic table could be virtually separated by this process.
Back in 1940s the solvent extraction was primarily used to separate nuclear and rare earth elements.
However, availability of inexpensive and effective reagents led to the establishment of large-scale solvent extraction processes for extraction of non-ferrous metals from hydrometallurgical leach liquors.
Solvent extraction consists of transferring one or more solutes contained in a feed solution to another immiscible liquid (solvent), the solvent that is enriched in solute is called Extract and the feed solution that is depleted of solute is called Raffinate
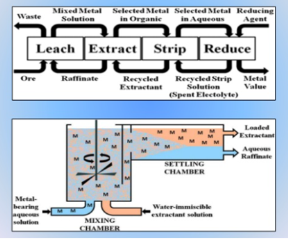
Fig 14: Solvent extraction as a part of Hydrometallurgy
Types of Solvent Extraction
Solvating Extraction
Cationic Exchange
Anionic Exchange
Chelating Extraction
Two Basic steps of Solvent extraction
1. Extraction
UO2(SO4)4- + 2(R3NH)2SO4 = (R3NH)4UO2(SO4)3 +2(SO42-)
2. Stripping
(R3NH)4 UO2 (SO4)3 + Na2CO3 = R3N +Na4UO2(CO3)34- + H2O + Na2SO4
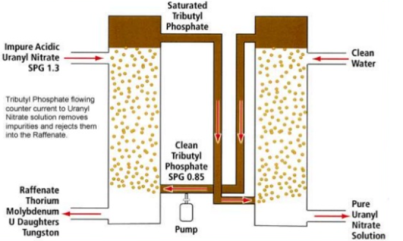
Fig 15: Solvent Extraction of Uranium
Single stage Solvent extraction
This process is commonly followed in the labs, contacting is carried out by taking two phases together in a separating funnel followed by vigorous agitation so that the phases may disperse in each other as fine droplets. Ceasing the agitation lead to the separation of phases in two distinct layers.
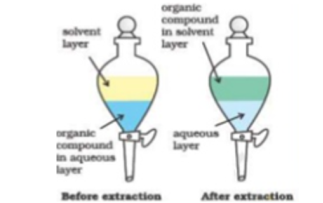
Fig 16: Single stage extraction
Multi Stage Counter Current Solvent Extraction
Applications
Today the process of solvent extraction is widely applied in miscellneous fields of sceince and techology
The hydrometallurgical processing of complex ores/ concentrates/ secondaries/ wastes, the solutions containing different non-ferrous metals are obtained during leaching the materials in acidic, ammoniacal or alkaline lixiviants. The spent solutions and effluents are also generated in different process industries and contain metallic values. Their discharge in the sewage or river is major concern for environment. Solvent extraction (SX) is one such proven technique in the hydrometallurgical processing for selective extraction and separation of metals due to the ease of applicability, versatility and ability to produce high value products. It is used on commercial scale for the recovery of different metals from different solutions viz. copper, nickel, cobalt, zinc, tungsten molybdenum, uranium, rare earths etc. The effluents from waste streams are also processed to recover metals using organic extractants. In the solvent extraction process, the extraction of metal ions, or uncharged species in the aqueous phase takes place by ion pair transfer, ion exchange with the extractant. In the case of ion-pair transfer, electrically neutral molecules interact with the extractant to form an addition compound. The most suitable extractants for such interaction are those having an oxygen atom with a lone pair of electrons viz. ether, alcohols, and the neutral phosphoric acid esters. For example, tri-butyl phosphate extracts uranium from nitric acid solutions as follows:

Similarly, the metal is transferred from the aqueous phase as simple ion, and at the same time an ion from the extractant is transferred stoichiometrically to the aqueous phase in the ion exchange process. The ion present in the aqueous phase may be either cationic or anionic form. The extraction of metal takes place by cation or anion exchange mechanism.
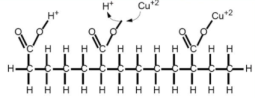
Fig 17: Carboxyl groups exchanges the ion it currently holds (H+) for a Cu2+ ion, the Cu2+ is later released by contacting it with a stripping solution (very high H+ conc.)
Oxidative refining
In metallurgy, refining process against impurities refers to the purification of impure metal, in this process, the end product is chemically identical to the original material but purer in form
Reduction method of metals cannot produce the purest version of metals, in such cases, refining technique assists to obtain the 100% pure metal in small quantity by removing impurities.
However, this technique varies with different metals, sometimes a specific substance helps to bring out the desirable form of a metal. In some cases, a particular metal is refined to acquire some valuable by-products.
Oxidation or Cupellation
It is a process of removing impurities from silver or gold in a cupel ( a shaloe porous dish made of bone ash or other refractory material).
In this process, te impure gold or silver stays on the cupel and then a blast of hot air passes through it within a special furnace. The impurities like tin,copper,lead etc are oidised.The cupel absorbs portions of the impurities and the rest vapourises, leaving behind the pure silver or gold.
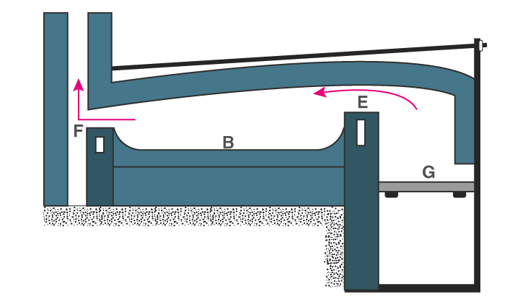
Fig 18:Cupellation of Gold
Zone refining
The impurities are higher solubility in the molten metal as compared to that in solid metal, the difference in solubility of impurities makes this process possible.
Zone refining is a technique to get highly pure crystals of impure elements (specially metals) by using melting and crystallization processes. In this narrow region of the crystal is heated so that impurities get melted and forms a molten zone which moves along the crystal.
Principle of zone refining
Zone refining technique is based on the fact that impurity has more solubility in molten state, because of this when a molten metal crystallizes on cooling, impurities are automatically excluded as they do not form part of the pure crystal.
In zone refining, impurities are removed by moving the molten zone through heater and recrystallized pure metal is left behind in solid form, in this zone should be moving slowly as possible to get highly pure metal.
Zone Refining process
In the zone refining process,a mobile heater is fixed at one end of te impure metal rod which is to be purified, now an impure metal rod fitted with a circular mobile heater is fixed in a column filled wth inert gas.Tis circular heater heats the rod radially and produces a region in which the temperature is uniformly elevated to the melting point I a plane perpendicular to the axis of the rod. As the heater moves along the rod, the molten zone also propagates down the rod. We can make the heater move very slowly, because of this impurities and pure metal both get melted but atoms of pure metal recrystallize while molten impurities move with heater or molten zone, thus after the process impurities gets collected at one end while solid pure metal remains at another end, the process is repeated many times to get pure metal.
Applications of Zone refining
Limitations of Zone refining
Zone refining is an expensive process, because of tis applications are limited to lab reagents and valuable chemicals. Sometimes when solid-liquid equilibria are not favourable for all impurities then we have to combine zone refining with the techniques to achieve ultra-high purity.
Scientist W.G Pfann discovered this refining process against impurities, typically metals that are purified using this method are gallium, silicon, indium, germanium, boron and other metals.
The impurities are higher solubility in the molten metal as compared to that in solid metal, the difference in solubility of impurities makes this process possible.
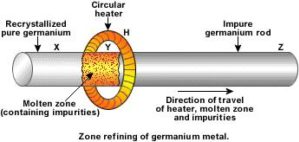
Fig 19: Zone refining
Kroll’s process
Titanium was first discovered in the 1700s, but was not identified and given a name until the early 1900’s.
The word Titan and its metal alloy has led to the name of titanium and has lived up to its name, it has been the driving force for much of the world’s technology and equipment.
The metal alloy is found in the earth in plenty and the last few decades it is easily extracted from earth. Crude titanium ore cannot be used in its natural form. It must be taken through a tedious and time-consuming process that uses dangerous chemicals and costly equipment.
The process of transforming crude titanium from the earth (known as ore) to the type of metal we use in many aspects of our lives is called the Kroll Process.
The Kroll Process is named after William J. Kroll, who invented the process in 1940 to produce Zirconium
The Kroll Process is comprised of 6 steps to produce the titanium we have come to use and benefit from:
Step 1: The pure titanium is changed into sponge by passing an electric charge through the ore, this process occurs in a chlorinator, the chlorine gas is then passed through a charge.
Step 2: Titanium tetrachloride is formed by removing an oxygen resulting in the formation of titanium tetrachloride. This crude form of titanium tetrachloride is then purified through fractional distillation.
Step 3: Once distillation is complete, magnesium or sodium is added to the pure titanium tetrachloride to form a metallic sponge and either magnesium chloride or sodium chloride is liberated.
Step 4: The newly formed metallic sponge is then crushed and pressed.
Step 5: The crushed titanium sponge is then melted in an electrode vacuum arc furnace at extremely high temperatures.
Step 6: Each batch presenting the furnace is known as ingot and weighs up to 12000 pounds, the melted titanium is allowed to harden and solidify in the furnace rather than being poured out.
Titanium was once flaunted as the metal that belongs to the future, it has played a major role in various advancements in the field of aerospace, defence, manufacturing, medical, transportation and leisure industries.
The comfort and luxury we enjoy today would not be possible without the discovery of Kroll’s process.
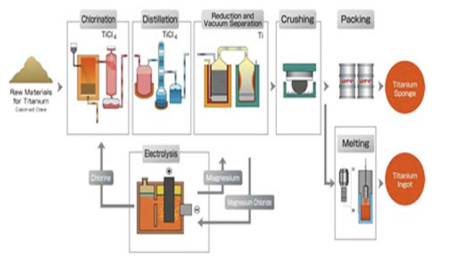
Fig 20: Kroll’s process
Van Arkell de Boer method
The process was first discovered by a Dutch chemist Anton Eduard van Arkell in 1952, the process is used to produce pure metal like tungsten, titanium and zirconium by forming metal iodides followed by thermal decomposition on a hot tungsten.
It is used in the purification of titanium and zirconium, impure Ti/Zr is heated in ana evacuated vessel with iodine, the volatile titaniumtetreiodide/zirconium tetraiodide is the final product which is finally heated with a tungsten filament to acquire pure metal.
The process is also known as iodide process or the crystal bar process, this process was the first industrial production of ductile metallic zirconium in its pure form, this is used in small quantites for the production of ultra pure zirconium and titanium. The process succeeded the kroll process.
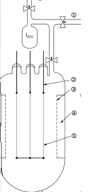
Fig 21:An apparatus used for the crystal bar process.
The main body is made of quartz glass. (1) to vacuum pump, (2) 6 mm molybdenum electrode, (3) molybdenum net, (4) chamber for the raw metal, (5) tungsten wire
The impure form of thorium, protactinium, vanadium, hafnium, titanium and zirconium are heated in an evacuated vessel along with a halogen at a temperature of 50-250oC. the process clearly depicts the involvement of Til4 and Zrl4, which become volatile leaving behind the impurities s solid. At atmospheric pressure TiI4 melts at 150 °C and boils at 377 °C, while ZrI4 melts at 499 °C and boils at 600 °C. At reduced pressure the boiling points are low. The gaseous metal tetraiodide is decomposed on a white-hot tungsten filament (1400 °C). As more metal is deposited the filament conducts better and thus a greater electric current is required to maintain the temperature of the filament. The process can be performed in the span of several hours or several weeks, depending on the particular setup.
Can take place using a number of metals and also any halogen or a combination of halogens that is appropriate for the transport mechanism and the reactions involved. The titanium, zirconium and hafnium are the only metals used on an industrial scale due to its pure form. They are used extensively in small scale industries for specific purity needs.
Mond’s Process
The Mond process is a method for purifying nickel. In this lesson we will learn what this process is, how it works, and what applications it is used for.
One of the common types of rechargeable batteries uses nickel as the main ingredient to allow it to work. But nickel that is simply mined doesn't work, because it isn't pure nickel. Thus, it needs to be refined. One process for refining nickel is the Mond process (also called the carbonyl process)
The Mond process was developed by Ludwig Mond, a German-British chemist, in the late 19th century. He discovered that metals can form carbonyls under specific conditions, and thus discovered methods for refining metals
The Reaction
There are three reaction steps in the Mond process. Nickel typically comes in the form of nickel oxide, with other impurities. In step 1, syngas, which is a mixture of hydrogen gas and carbon monoxide gas, is added to the nickel. The nickel oxide and impurities react with the hydrogen gas to form an impure solid nickel:

Although the reaction makes it appear as though we have pure nickel, it is not pure at this point
Next, the carbon monoxide will react with the impure nickel. But only nickel will readily react with the carbon monoxide - the other metals won't. The nickel and carbon monoxide reacts to form nickel carbonyl gas, but the impurities remain as solids:

Finally, the nickel carbonyl gas is heated, causing the nickel carbonyl to decompose (break apart) into pure nickel and carbon monoxide:

Them the carbon monoxide is recycled to continue the process and the nickel is collected.

Fig 22: Nickel is collected from the Mond process
This process works using a method called vapor phase refining, where two compounds combine to form an easily volatile substance. But the two compounds can easily decompose when heated, and then the pure metal can be collected.
Li (Lithium)
Uses of Lithium
Properties of Lithium
Be (Beryllium)
Beryllium is the lighest member of the family of alkaline earth etal and has an atomic number 4 and belongs to group 2,period2 and s-block in the periodic table.Beryllium is a divalent and its compounds are highly toxic and carcinogenoc. It is silvery-white metal with low density and relatively soft, it occurs in more than 30various mineral species among whixh the most imporatnt ones are beryl,bertrandite and phenacite. Precious form of beryl is emerld and aquamarine, Beryllium mass number is 9.01218g/mol and has the symbol”Be’’. The state of beryllium is solid at 20oC and has a configuration 1s22s2
USES
Discovery
French Louis-Nicholas Vanquelin discovered the element berylium in 1798, it was first called glucinium which means sweet as all the compounds of beryllium tasted sweet, on the basis of a gemstone , beryllium got its name.
Physical properties
Chemical properties of beryllium
Beryllium compounds
Compounds of beryllium have an exclusive oxidation state of +2, these compounds are colourless and can lead to dermatitis due to toxic fumes which is hypersensitive to berylliosis but sweet in taste, even being a normal compound is considered as an electrical insulator because of its poor conductivity towards the electric current, they are also good conductors of heat.
Ra (Radium)
Radium is a chemical element with symbol Ra. It is the sixth element in the Group 2 of the periodic table
Pure radium is silvery-white in colour, but it combines with nitrogen readily on exposure to air and forms a black surface layer of radium nitride.
Radium was discovered by Marie Sklodowska Curie and Perre Curie in 1898, in the form of radium chloride. They extracted the radium compound from uraninite. It is found in uranium ores at 1 part per 3 million parts uranium.
Physical properties of Radium
Radium is the heaviest known alkaline earth metal and is the only radioactive member of its group. Its physical and chemical properties most closely resemble its lighter congener barium.
Radium is a highly reactive metal and always exhibits its group oxidation state. It forms the colorless Ra2+ cation in aqueous solution, which is highly basic and doesn’t form complexes. Most radium compounds are therefore simple ionic compounds.
Radium oxide: RaO
Radium Hydroxide: Ra(OH)2
Radium chloride: RaCl2
Radium bromide: RaBr2
Radium Nitrate: Ra(NO3)2
Radium emits alpha, beta, and gamma rays when mixed with beryllium produces neutrons.
Chemical Properties of Radium
Group | 2 | Melting point | 696°C, 1285°F, 969 K |
Period | 7 | Boiling point | 1500°C, 2732°F, 1773 K |
Block | s | Density (g cm−3) | 5 |
Atomic number | 88 | Relative atomic mass | [226] |
State at 20°C | Solid | Key isotopes | 226Ra |
Electron configuration | [Rn] 7s2 | CAS number | 7440-14-4 |
ChemSpider ID | 4886483 |
|
|
Applications and effects of Radium
Health effects of Radium
It is highly radiotoxic and carcinogen by inhalation, ingestion or exposure and used in treating cancer and other body disorders. The element Ra is over a million times more radioactive than the same mass of uranium
(b) Sn, Pb.
Sn(Tin)
Tin or also called as Stannum in Latin with the atomic number 50 belongs to the group 14 of the periodic table.
Tin shows a chemical similarity to both of its neighbours in group 14, germanium and lead, and has two main oxidation states, +2 and the slightly more stable +4.
Uses of Tin (Sn element)
Properties of Tin
Allotropes of Tin (Sn element)
Health Effects of Tin
Pb(lead)
Lead (pronunciation: LED) it’s a soft ductile malleable element that has corrosion resistance, and has the symbol Pb. Lead is a poor conductor of electricity, when exposed to air if forms a dull coating, they belong to the family of post-transition metals, it has 35 isotopes with mass numbers ranging from 181 to 125 of which Pb-206, Pb-207, and Pb-208 have a stable half-life.
Pb is present as a free metal that consists of 0.0013% of the earth’s crust and also obtained from various minerals ores like galena, cerussite, minum, anglesite. In the US, through the roasting process, one-third of the element is extracted from galena, other sources include scrap batteries and pipes that contributes to 40%of produce in the UK carried out in lead smelters.
Origin of its Name: It is named after the Anglo-Saxon word ‘lead’ while its symbol Pb is derived from the Latin word ‘plumbum’, meaning waterworks
Classification, Properties and Characteristics of Lead
General Properties | |||
Relative atomic mass | 207.2 | ||
Atomic mass/weight | 207.2 atomic mass units | ||
Molar mass | 207.2 g/mole | ||
Mass number | 207 | ||
Physical Properties | |||
Color/physical appearance | Silver-grey | ||
Melting point/freezing point | 327.462°C (621.432°F) | ||
Boiling point | 1749°C (3180°F) | ||
Density | 11.3 g/cm3 | ||
Standard state at room temperature (solid/liquid/gas) | Solid | ||
Malleability | Yes | ||
Ductility | Yes | ||
Hardness | 1.5 Mohs | ||
Specific heat capacity | 0.3 J g-1K-1 | ||
Magnetic Properties | |||
Magnetic type | Diamagnetic | ||
Molar magnetic susceptibility | -3.11×10-10 m3/mol | ||
Mass magnetic susceptibility | -1.5×10-9 m3/kg | ||
Volume magnetic susceptibility | -0.000017 | ||
Chemical Properties | |||
Flammability | Unknown | ||
Oxidation states (numbers) | 4, 2 | ||
What is Lead Used for
Lead is a corrosion resistance metal, used in the manufacturing of pipes paints, hair dyes etc. They are also added to petrol as an anti-knocking agent to improve the performance of engine. However, these uses have been limited due to the toxic effects of Pb.
Batteries with lead acid are utilised to operate automobiles, submarines and emergency generators have a long life and low impedance.
The element is applicable in the ammunition industry for making shots and bullets.
Lead-sheathed cables and wires are used in petrochemicals plants as very good chemical barriers against hydrocarbons and moisture.
It is used in pencils, flashings, lifting weights, anchors, and diving weight belts
Glasses coated with its oxide are used in making windows (known as lead lights) and roofing. Pb crystal glasses are sometimes utilized as storage containers for corrosive liquids.
Solder joints comprising of lead and tin in equal amounts are used in joining pipes and electrical components. There are other alloys of the metal as well used in printing plates, presses, and bearings.
Extraction
Halogens are extracted and recovered from natural brine, it is also concerned with the extraction and recovery of iodine from the naturally occurring brine that may contain minute amounts of iodine.
A well-known process for extracting iodine from natural brine is to acidify the brine, followed by oxidizing the acidified brine along with chlorine gas leading to the liberation of iodine in its elemental state.
The iodine is vapourised by blowing out iron by a current of air, thus separating the iodine vapours from the air stream either by absorbing them on a suitable medium like charcoal or a solvent or by dissolving them in an agent that combines with iodine.
The acidification of brine brings about a change in pH to a value of 2.5 or low , in order to avoid the excess loss of the product during ensuing oxidation and blow-out steps, If the pH value of the brine is not of the low order just mentioned prior to the oxidizing step, a considerable portion of the iodine first formed is further oxidized to lodate by the strong oxidizing action of the chlorine usually employed as the oxidizing agent; and since the iodate is readily soluble in the acid liquor, it is not blown out and thus recovered, but instead is carried out and lost in the waste tail liquors from the blowing out tower. by employing my improved process, the acidity of the brine during the oxidizing step can be maintained at a much higher pH value than is possible if chlorine is employed as the oxidizing agent. For example, when employing chlorine as the oxidizing agent it is necessary in practical operation of the process to maintain the acidified brine at a pH value of from 2 to 2.5, while if a hypochlorite solution is employed, in accordance with the present invention, the oxidizing step is most suitably carried out at a pH value of from about 3 to 6, and preferably at between about 4 and 5. It is essential to employ an amount of acid such as will bring the pH value of the brine to below about 6, for otherwise a satisfactory yield or recovery of iodine will not be obtained. On the other hand, an increased recovery of iodine is not obtained by increasing the acidity of the brine to a pH value below about 4 when employing a hypochlorite solution as an oxidizing agent, although such increased acidity does no harm as regards a good recovery of iodine. Operation at a pH value of between 4-6 results in a great saving in the total amount of acid consumed in the process, with a resultant economy of operation.
Among the hypochlorite’s that may be suitably used for carrying out the oxidation to liberate iodine are the hypochlorite’s of sodium, potassium, calcium, and magnesium, and hypochlorous acid
Oxidation states
The best sources of halogens (except iodine) are halide salts. There is a possibility to oxidise the halide ions to liberate the diatomic halogen molecules by various methods, which depends on the oxidation of the halide ion, Fluoride is the most difficult element to oxidise and iodide is the easiest.
The major method for preparing fluorine is electrolytic oxidation. The general procedure of electrolysis is to use a molten mixture of potassium hydrogen fluoride, KHF2, and anhydrous hydrogen fluoride. In the process the electrolysis causes HF to decompose, forming fluorine gas at the anode and hydrogen at the cathode. Here it is important to keep the two gases separated to stop their explosive recombination to reform hydrogen fluoride.
The electrolysis of Chlorine ion in the aqueous solution of sodium chloride, produces the commercial chlorine. Chlorine is also a product of the electrolytic production of metals such as sodium, calcium, and magnesium from their fused chlorides. It is also possible to prepare chlorine by the chemical oxidation of the chloride ion in acid solution with strong oxidizing agents such as manganese dioxide (MnO2) or sodium dichromate (Na2Cr2O7). The reaction with manganese dioxide is:
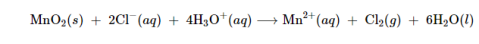
The commercial preparation of bromine involves the oxidation of bromide ion by chlorine
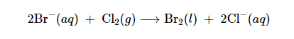
Some iodine comes from the oxidation of iodine chloride, ICl, or iodic acid, HlO3. The commercial preparation of iodine utilizes the reduction of sodium iodate, NaIO3, an impurity in deposits of Chile saltpeter, with sodium hydrogen sulfite:
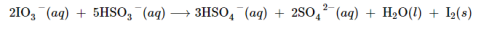
Halides
Halides are binary compounds that are of which one part is an element another part is the halogen atom. A radical is less electronegative compared to that of halogen which form astatine, bromide, fluoride and chloride. Most of the salts are halides. Various halide compounds are tested using silver nitrate solution. Some include Kl, KBr, and KCl.
When halogen reacts with silver nitrate solution, precipitation will be formed, and it varies in colour depending upon the type of halides. Some include Silver Fluoride with no precipitate. Silver Bromide with pale yellow precipitate. Silver Iodide with green precipitate. Silver Chloride with a white precipitate.
Halogen Atom comprising a negative charge is termed as the halide ion. Halide mineral includes halide anion. Fluorite and Halite are two important halide minerals. Fluorite is the main source of hydrogen fluoride. Halite is a primary source of sodium chloride. Bischofite forms a primary source of magnesium. Many of the halides are present in the marine evaporite deposits. Few of the halide anions include iodide, bromide, chloride, and fluoride.
Example of Halide Compounds
Organic Halide compounds consist of one or more halogens and belong to a class of synthetic and natural chemicals. Some examples of halide compound include calcium chloride, silver chloride, potassium iodide, potassium chloride, sodium chloride, Iodoform, Chlorine Fluoride, Organohalides, Bromoethane and more.
Metal Halides
Metal Halides are compounds between a halogen and metals. Some are covalently bond, and some are ionic. Covalently bonded metal ions may form polymeric structures. Metal Halides are formed when all halogens react with metal. It is stated in the below equation.
2M+nX2→2MXn
Uses of Halides
Key take away
References: