Unit – 1
Water Chemistry
Water is a chemical compound consisting of two hydrogen atoms and one Oxygen atom. The name water typically refers to the liquid state of the compound. The solid phase is called as ice and the gas phase is called as steam. Under specific conditions, water also forms a supercritical fluid. Water is the main compound found in living organisms. Approximately 62 percent of the human : body contains water. The word "water" comes from the Old English word water or from the Proto-Germanic watar or German Wasser. All of which mean "water" or "wet." The boiling point of water is 99.98 degrees C (211.96 degrees F; 373.13 K).
Water is amphoteric. Which means, it can act as both an acid and as a base.
Hard and soft water:
Hard water: Hard Water is water that contains a required quantity of dissolved minerals (like calcium and magnesium) As rainwater falls, it is naturally soft. However, as water flows through the ground and into waterways, it picks up minerals like chalk, lime and mostly calcium and magnesium and becomes hard water. Since hard water contains essential minerals, it is essentially used as drinking water. Not only because of the health benefits, but also the flavour. Water that does not produce lather with soap readily is called hard water. Water hardness is usually measured as calcium hardness in milligrams per litre (mg/l) OR parts per million (ppm) OR in grains per gallon (GPG).
For e.g.: sea water, river water, spring water, lake water and well water.
Soft water: water that shows the absence of dissolved salts of such metals as magnesium, iron, or calcium, which are known to form insoluble deposits such as appear as scale in boilers or soap curds in bathtubs and laundry equipment, soft water is neither healthy nor desirable to drink. . Water that readily produces lather with soap is called soft water.
For e.g.: Rain water, distilled water, demineralised water.
1.1.2 Water quality parameters like pH, Acidity, alkalinity, total solids, Dissolved oxygen, Chlorides
There are three types of water quality parameters physical, biological and chemical. The Physical parameters of water quality include:
Turbidity:
Turbidity is the cloudiness present in water. It is a measure of the ability of light to pass through water. It is caused by suspended materials such as silt, clay, plankton, organic material, and other particulate materials present in water.
Turbidity in drinking water is aesthetically unacceptable, which makes the water look unappetizing. The impact of turbidity can be summarized in the following points:
It can increase the cost of water treatment for various uses.
The particulates can shelter harmful microorganisms and thereby protect them from the disinfection process.
Suspended materials can clog or damage fish gills, decreasing its resistance to diseases, reducing its growth rates, affecting egg and larval maturing, and affecting the efficiency of fish catching method.
Suspended particles provide a medium of adsorption for heavy metals such as cadium, lead, chromium, mercury, and many hazardous organic pollutants such as polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), and many types pesticides.
The amount of available food is reduced because higher turbidity raises water. Consequently, the concentration of the dissolved oxygen (DO) can be decreased since warm water carries less dissolved oxygen compared to cold water.
Turbidity can be measured by an instrument called Nephelo metricturbidi meter, the instrument expresses turbidity in terms of NTU or TU. A TU is equivalent to 1 mg/L of silica in suspension.
Turbidity that is more than 5 NTU can be visible to the average person while turbidity in muddy water, it exceeds 100 NTU. Groundwater normally has very low turbidity because of the natural filtration that occurs as the water penetrates through the soil.
Temperature
Palatability, viscosity, solubility, odours, and chemical reactions are influenced by temperature. Thereby, the chlorination and sedimentation, processes and biological oxygen demand (BOD) are dependent on temperature.
Colour
Materials decayed from organic matter, such as any, vegetation and inorganic matter namely stones, soil, and rocks impart colour to water, which is objectionable for aesthetic reasons, not for health reasons.
Colour is measured by comparing the water sample with standard colour solutions or coloured glass disks. One unit of colour is equivalent to the colour produced by a 1 mg/L solution of platinum (potassium chloroplatinate (K2PtCl6)).
The colour of a water sample can be reported as follows:
Apparent colour is the entire water sample colour and consists of both dissolved and suspended components colour.
True colour of the water sample is measured after filtering the water sample to remove all suspended particles.
Colour is graded on scale of 0 (clear) to 70 colour units. Pure water is colourless, which is equivalent to 0 colour units.
Taste and odour
Taste and odour in water can be caused by foreign materials such as organic materials, inorganic compounds, or dissolved gasses. These materials arise from natural, domestic, or agricultural sources.
The numerical value of odour or taste is determined quantitatively by measuring a volume of sample A and diluting it with a volume of sample B of an odour-free distilled water so that the odour of the resulting mixture is just detectable at a total mixture volume of 200 ml. The unit of odour or taste is expressed in terms of a threshold number as follows:
TON or TTN = (A + B)/A
Where TON is the threshold odour number and TTN is the threshold taste number.
Solids
Solids exist in water in either forms, a solution or in suspension. The two types of solids can be identified by using a glass fiber filter through which water sample passes through. By definition, the suspended solids are retained on the top of the filter and the dissolved solids pass through the filter with the water.
When the filtered portion of the water sample is placed in a small dish and then evaporated, the solids form as a residue. This material is called total dissolved solids or TDS.
Total solid (TS) = Total dissolved solid (TDS) + Total suspended solid (TSS)
Water can be classified by the amount of TDS per liter as follows:
Freshwater: <1500 mg/L TDS;
Brackish water: 1500–5000 mg/L TDS;
Saline water: >5000 mg/L TDS.
The residue of TSS and TDS after heating to dryness for a specified period of time and at a specific temperature is defined as fixed solids. Volatile solids are those solids lost on ignition (heating to 550°C).
These necessary measures help the operators of the wastewater treatment plant because they roughly approximate the amount of organic matter that exists in the total solids of Industrial waste, activated sludge, and wastewater.
Total solids:
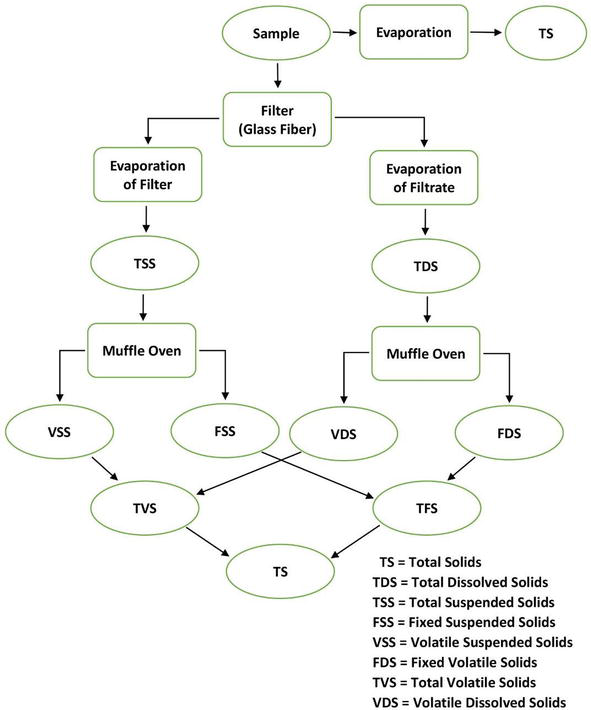
Figure 3: Interrelationship of solids found in water.
Total solids (mg/L) = [(TSA – TSB)] × 1000/sample(mL)
Acidity
The acidity and basicity of the water measured over the pH scale. PH of solution is taken as –ve logarithm of H2 ions for many practical practices. Value range of pH from 7 to 14 is alkaline, from 0 to 7 is acidic and 7 is neutral. Mainly drinking water pH lies from 4.4 to 8.5. The pH scale commonly ranges from 0 to 14.
Alkalinity:
A natural water may be alkaline due to presence of hydroxide bicarbonates and carbonates compound dissolve in water.
Hydroxides 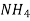 OH, NaOH
OH, NaOH
Bicarbonates Ca (HCO3)2
Carbonates MgCO3 , FeCO3
Hydroxides and carbonates and stronger bases than bicarbonates.
- When an alkaline water is titrated with a strong acid first all OH get neutralized then all the caco3 – ions are half neutralized + OHCo3- .
- Till this stage, ph of mixture decreases to about 8.2 and completion of this stage is indicated by change in color of phenolphthalein.
- On continued addition of acid during titration all the HCO3 in the titration mixture (produce by half neutralization of CO3 and present from beginning) get neutralized and completion of this stage is indicated by methyl orange color change at about3.7 ph.

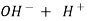
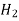 O (PH = 8.2)
O (PH = 8.2)
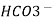 + H+
+ H+  H2O + CO2 ( PH = 3.7 0)
H2O + CO2 ( PH = 3.7 0)
Procedure :
The alkalineties due to the three type of ions can be easily determined by neutralisation titration.
- Take V ml ( generally 25 ml ) of the alkaline water in conical flask and add 2 drops of phenopthalein indicator in it .
- Titrate this sample against standard strong acid solution ( x n ) from burette till pin k colour changes to colourless . Klet the burette be V 1 ml .
- Add few drops of methyl orange indicator into the same titrarting mixture changes to orange.
Note the burette reading as V 2 ml ( from initial )
Calculations :-
P = phenolphthalein alkalinity = 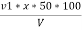
= PPM Caco3 equivalent
M = methyl orange alkalinity = total alkalinity
= 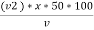 ppm Caco3 equivalent
ppm Caco3 equivalent
The possible combinatuions of alkalinites in water are:-
- Only OH-
- Only HCO3-
- ONLY CO3-
- OH- and CO3 – Together
Chloride
Chloride ions amount in water sample ( bymoho’s method)
- Under ground and surface water are rich with cl- in the form of Nacl, Kcl , etc. .
- Their quantity over 250 mgl liter imparts bad taste to water. Mgcl2 , Cacl2 cause hardness and being the salts of weak base strong acid are harmful for industry and boiler use .
Theory
- Cl – quantity in a water sample is found precipitation titration method. ( mohor’s method )
 In the mohor’smethod,
In the mohor’smethod,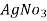 is the titrant and potassium chromate as indicator in the titration.
is the titrant and potassium chromate as indicator in the titration.
 ( Ksp = 1.82 *
( Ksp = 1.82 * 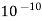 )
)
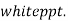

 ( ksp = 1.1 *
( ksp = 1.1 * 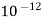 )
)
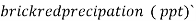
3. When Ag + ions are added to the mixture container of an indicator Cro4 there is first formation of Agcl, although Agcl has lesser solubility product than Ag2Cro4.
4. The reason for why Ag2Cro4 precipitate formation requires excess concentration of Ag++ to exceed its kip.
Procedure: -
- Take 50 ml of a chloride water sample in a conical flask and add a pinch of caco3 to neutralize the water if it is acidic.
- Add few drops of a potassium chromatic indicator solution into the water and slowly add Agno3 solution (z molarity) from burette.
- Note the end point when permanent reddish tinge is obtained. Let the burette reading by Y ml.
Calculations: -
 volume of
volume of 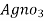 for 50 ml water sample = Y ml
for 50 ml water sample = Y ml
∴volume of 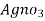 for 1000 ml water sample 1000/50 * y ml
for 1000 ml water sample 1000/50 * y ml
= 20 y ml
∴ 1000 ml 1 m 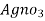 = 35.5 * 1000 mg cl
= 35.5 * 1000 mg cl
∴ 20 y ml 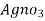 =
= 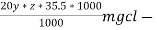
= 20yz * 35.5 mg cl- / litre
Dissolved oxygen (Definition, Causes, Significance)
Adequate dissolved oxygen concentrations are critical during all phases of striped bass and hybrid culture. Low dissolved oxygen concentrations can result in slower growth and induce the stress response predisposing the animals to infectious disease. Monitoring of dissolved oxygen concentrations is complicated by the rate at which they can change. In heavily stocked raceways, tanks, or flow-through systems, for example, an interruption of oxygenation may result in critically low dissolved oxygen concentrations within minutes due to consumption by the culture animals. Management of dissolved oxygen concentrations in ponds must also consider the daily rhythms of concentrations characteristic of ponds. Striped bass and its hybrids have different dissolved oxygen requirements at different stages in their lives. Striped bass also appear to require higher concentrations of dissolved oxygen relative to other temperate species. Generally, dissolved oxygen concentrations should be maintained as close to saturation as possible for best survival and growth.
1.1.5 BOD, COD- definition, significance and Numerical problems:
Biochemical Oxygen Demand:
The determination of the Biochemical Oxygen Demand or Biological Oxygen Demand (BOD) evaluates the amount of biodegradable organic material present in wastewater, effluent and polluted waters. The BOD test reflects the amount of dissolved oxygen (DO) consumed by bacteria while oxidizing these materials. Dissolved oxygen is essential for the life of aquatic fauna and flora, and the BOD test is a measure of the ecological impact that effluent water may have on the receiving body of water (river, lake, etc.). This test is often required in discharge permits, as it is a means of assessing the degree of water pollution.
Two methods are widely used for BOD measurement: the dilution method and the manometric method:
- Dilution method
This is a standard method. As an example, the APHA standard methods 5210B from the American Public Health Association describes in detail this protocol. Dilution water is prepared by adding inorganic nutrients and buffer salts to purified water. If the sample does not contain sufficient amounts of bacteria, or contains compounds toxic to bacteria (e.g. Chlorinated effluent), it may be necessary to add microbial seed as well. Various dilution levels of the sample water are then prepared using the dilution water. The BOD bottles are filled to the top, capped and sealed. They are incubated in the dark at 20°C for 5 days. The levels of dissolved oxygen are measured prior to and after the 5-day incubation period. The difference between these two values, corrected for the dilution and the blank, is the BOD5 value. BOD tests results are expressed in mg/L of dissolved oxygen. - Manometric method
In this test, a manometer is fitted into a bottle containing the undiluted sample. It continuously measures the drop in air pressure in the bottle, which reflects the amount of oxygen uptake by the sample. This method is easier than the dilution method because no dilution is necessary, and continuous measurements are obtained.
The presence of toxicants or poor seeding material in water samples may lead to falsely low BOD results. Therefore, it is recommended to regularly use a glucose - glutamic acid (GGA) solution as a standard check solution. The oxygen uptake of this solution should be 198 +/- 30.5 mg/L.
Chemical Oxygen Demand: The chemical oxygen demand (COD) is a measure of water and wastewater quality. The COD test is often used to monitor water treatment plant efficiency. This test is based on the fact that a strong oxidizing agent, under acidic conditions, can fully oxidize almost any organic compound to carbon dioxide. The COD is the amount of oxygen consumed to chemically oxidize organic water contaminants to inorganic end products.
The COD is often measured using a strong oxidant (e.g. Potassium dichromate, potassium iodate, potassium permanganate) under acidic conditions. A known excess amount of the oxidant is added to the sample. Once oxidation is complete, the concentration of organics in the sample is calculated by measuring the amount of oxidant remaining in the solution. This is usually done by titration, using an indicator solution. COD is expressed in mg/L, which indicates the mass of oxygen consumed per liter of solution.
COD is a second method of estimating how much oxygen would be depleted from a body of receiving water as a result of bacterial action. The COD test uses a strong chemical oxidizing agent (potassium dichromate or potassium permanganate) to chemically oxidize the organic material in the sample of wastewater under conditions of heat and strong acid. The COD test has the advantage of not being subject to interference from toxic materials, as well as requiring only two or three hours for test completion, as opposed to five days for the BOD test. It has the disadvantage of being completely artificial, but is nevertheless considered to yield a result that may be used as the basis upon which to calculate a reasonably accurate and reproducible estimate of the oxygen-demanding properties of a wastewater. The COD test is often used in conjunction with the BOD test to estimate the amount of non-biodegradable organic material in a wastewater. In the case of biodegradable organics, the COD is normally in the range of 1.3 to 1.5 times the BOD. When the result of a COD test is more than twice that of the BOD test, there is good reason to suspect that a significant portion of the organic material in the sample is not biodegradable by ordinary microorganisms. As a side note, it is important to be aware that the sample vial resulting from a COD test can contain leachable mercury above regulatory limits. If such is the case, the sample must be managed as a toxic hazardous waste.
1. Parts Per Million (ppm):- is the parts of CaCO3 equivalent hardness per 106 parts of water i.e., 1ppm= 1 part of CaCO3 equivalent hardness in 106 parts of water.
2. Milligrams Per Litre (mg/L):- number of milligrams of CaCO3 equivalent hardness present per liter of water.
1mg/L=1mg of CaCO3
Equivalent hardness of 1L of water= 1kg=1000g=106mg.
∴1mg/L=1mg of CaCO3 eq per 106 mg of water=1ppm.
3. Clarke’s degree (0Cl):- the no. Of grains (1/7000lb) of CaCO3 equivalent hardness per gallon (10lb) of water or it is parts of CaCO3 equivalent hardness per 70,000 parts of water.
∴10Cl= 1 grain of CaCO3 eq hardness per gallon of water
= 1 part of CaCO3 hardness eq per 105 parts of water.
4. Degree French ( o Fr):- parts of CaCO3 equivalent hardness per 105 parts of water.
∴10 Fr= 1 part of CaCO3 equivalent hardness per 105 parts of water.
5. Milli-equivalent per liter (meq/L):- is the number of milli-equivalents of hardness present per liter.
1meq/L= 1meq of CaCO3 per liter of water
= 10-3 x 50 g of CaCO3 eq. Per liter
= 50 mg of CaCO3 eq. Per liter
= 50 mg/L of CaCO3 eq.
= 50ppm.
Types of hardness: -
- Temporary hardness (carbonate) :-
- When water containing calcium and magnesium bicarbonates is heated, bicarbonate decompose and from insoluble carbonate and form hydroxide.
- On filtering such water, soft water is obtained.
- The hardness which can be removed by more boiling is referred as ‘temporary hardness ’ or bicarbonate hardness.

 Ca
Ca

Mg 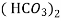
 Mg
Mg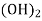 + 2 CO
+ 2 CO 
(Bicarbonates)
II. Permanent hardness :-
- The term permanent hardness or non-carbonate is the term applied to the hardness caused by dissolved chlorides, nitrates and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- Sum of temporary and permanent hardness is referred to as total hardness.
III. Alkaline or carbonate and non – alkaline or non – carbonate hardness :-
- Like all carbonate and bicarbonate, calcium and magnesium carbonate and bicarbonate are alkaline.
- Then hardness due to the carbonate and bicarbonate is called alkaline hardness or carbonate hardness.
- The alkalinity can be measures by titration with standard mineral acid using methyl orange and or phenol phthalein as an indicator.
- As the sulphate and chloride are neutral salts, the hardness caused by presence of calcium and magnesium sulphate, chlorides and nitrates are termed as non alkaline hardness or non carbonate hardness.
IV. Total hardness :-
The hardness due to all hardness causing salts, known as total hardness.
Total hardness = temporary + permanent
Estimation of hardness: -
Hardness of weather can be determined by two methods.
1) Soap solution method :-
- Total hardness of water can be determined by titrating a fixed volume of water sample (100ml) against standard alcoholic soap solution.
- Appearance of stable lather which persists for two minutes is the end point of titration.
- In the beginning sodium soap will precipitate all the hardness causing metal ions in the form of their soap (card) and then it will form free lather.
- If same water sample is boiled for 30minutes and then titrated against same soap solution the titration reading corresponds to permanent hardness.
The difference between two measurements corresponds to the temporary hardness of water.
1.2.1Calcium carbonate equivalent hardness and its calculations
It is a general term to express the hardness concentration and other elements such as salts, in equivalent chemical terms to calcium carbonate. The term basically means the dissolved concentration in chemically equivalent to the declared calcium carbonate concentration.
It can be defined as an expression of the concentration of specified constituents in water in terms of their equivalents to calcium carbonate.
Equivalent Weights
Calcium (Ca) 20.04
Magnesium (Mg) 12.15
Calcium carbonate (CaCO3) 50.045
CALCULATING CALCIUM HARDNESS AS CACO3
The hardness (in mg/L as CaCO3) for any given metallic ion is calculated using Equation:
Calcium hardness (mg/L) as CaCO3 = Calcium (mg
Equivalent weight of CaCO3 equivalent weight of calcium
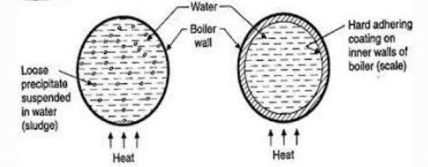
Sludge formation , scale formation
- In boiler water evaporates continously and the concentration of salts left behind goes on increasing . After the saturation point they get precipitated.
- If the precipitate remains in boiler tube as loose and slimy matter is called sludge.
- If some of the precipitated matter adhers strongly and forms strong bad conducting layer on their inner side of boiler tube , then it is known as scale.
Sludge :-( formation of sludge )
- The loose slimy mass of salts precipitated in boiler water is the sludge.
- They are generally formed at cooler portion of boiler and they loosely deposit in the parts of boiler tube where flow rate is slow e.g vlves bends
- Sludges are easy to remove by using brushes detergent solutions blow down opreation e.t.c .
Disadvanatages of sludges :-
- They tend to waste some portion of heat.
- Edcessive sludge formation distrub working of boiler and sometimes may choke up the pipe .
Prevention of sludges :-
- Use of water contaning very low quantity of total disolved solids.
- Frequently making blow down opreation i.e replacing salts concentrated water with fresh water.
Scales formation
Scale is the hard and strongly adhered coating to the inner surface of boiler and it is a bad conductor of heat. It is the main source of boiler trouble.
It is caused due to :-
- Decomposition of bicarbonates :-
At high temprature bicarbonates decompose into sticky water insolube material.
Ca ( HcO3)2 ----------------- CaCO3 + H2O + CO2
Mg ( HCo3)2 - - -------------- Mg (Oh)2 + 2CO2
2. Hydrolsis of magnesium salts :-
At higher temprature magnesium salts undergo hydrolysis.
MgCl2 + 2H2O ---------------------- mg ( OH)2 + 2HCl
3. Presence of silica :-
The source of ssilica is ( form) from sand and filter .silica may be in the form of colloidal particles. And it can be deposite as calcium silicate or magnesium silicate as firmly adhering materials.
4. Decreased solublity of CaSO4 :-
CaSo4 has lesser solublity at higher temprature hence at high temprature CaSo4 present in boiler feed water will precipitate as hard scale forming materials.
B] Disadvantages of scale :-
1. Waste of fuel :-
Scales are bad conductors of heat and resultes in the reduction of heat transfer to the boiler . Higher the thickness of scale greater than the wastage of fuel there by . It has been reported that 0.25 cm . Scale would increase fuel consuption by aboyut 2 to 3 percent.
Thickness of scale | 0.325 mm | 0.625mm | 1.25mm | 2.5 mm |
Wastage of fuel | 10 percent | 15 percent | 50 percent | 80 ercent |
3. Over heating of boiler :-
Scale being pooe conducter of heat it reduces transfer of heat from boiler to boiler water . To keep the required steam pressure we need to overheat the boiler .
4. Boiler saftey :-
Due to the scale formation the overheating of boiler is done in order to maintain constant stream supply with required pressure . This overheating makes boiler metal to become soft and weak . This cause distortation of boiler tube and becomes dangerous in morden high pressure boiler .
5. Danger of Explosion :-
When thick scale cracks due to uneven expansions the water come suddenl in contact with the overhead boiler metal. This cause large amount of steam formation suddenly and sudden high pressure is developed . Due to sudden high pressure is devloped . Due to sudden high pressure the softer boiler metal may burst with explosion.
C] Removal of scales :-
Scales are removeed from time by different ways.
- By use of suitable chemicals the scale can be dissolved and removed.
- Use of scraper or wire brush for thin scales to remove.
- Thick s ales may br removed by hammer and chisle.
- The thermal shocks technique is used to remove hard brittle scale . In this method empty boiler is heated and cooled by cold water suddenly . While sudden cooling the contracting boiler metal excerts pressure on scale to crack them.
- Blow down opreation used if scales are loosely adhering.
D] Prevention of scales :-
It is better to minimize scales formation and reduce the problems in steam generation.
- Use of softened water.
- Adding sodium phosphate to the water
- Frequent blow down operations to remove the scales when they are thin.
- Adding sodium aluminate which can trap the scale forming particles.
- Adding organic chemicals like tannin which forms coating on the scale forming particles . This matter becomes easily removable by blow down operation.
1.3.1 Softening of water by Ion exchange process and reverse osmosis process.
Ion exchange process:
Ion exchange technology is a proven method of producing high purity softened and demineralized water. It is used in most industries that require high purity water and to reclaim water from processes. The Ion exchange process involves the exchanging of contaminant ions for Na+ ions in a softening application and H+ and OH- ions in pure water application. Cations and anions can be removed by the cation and anion exchange resins. Resins containing –COOH, SO3H are capable for exchanging their H+ ions to cationic portion of minerals then it is called as cation exchanger while the resins containing –NH2, NHCH3 are capable for exchanging the anionic portion of the minerals then it is termed as anionic exchanger.
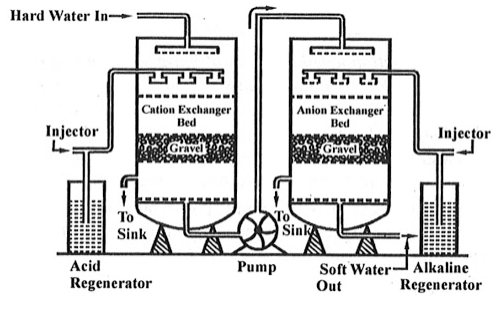
On supplying the hard water in first chamber which consists of Ca2+ or Mg2+ then the cation exchanger exchange it with H+ hence the cation exchanger absorbs the Ca2+ ions the left water are free from cations are passed to another chamber by the help of pump this water consists of anions such as Cl or SO4 on sprinkle up of these water at anion exchanger bed then it exchanged the anions and hence release the demineralise water. The absorbed cation and anion are sinked out through the outlet present in chamber.
Reverse Osmosis:
Reverse osmosis is a process where the water is separated from the salts in the source water by pressure-driven transport through a membrane. This process uses semi-permeable membrane and applied pressure to preferentially induce water permeation through the membrane while rejecting salts. The RO plant uses less energy than thermal desalination process. This process uses thin-film composite membrane that too comprises of ultra thin aromatic polyamide thin film. The used polyamide film gives the transparent properties while the remaining part provides the mechanical supports. The polyamide films are very dense void free polymer with high surface area allowing for its high permeability. RO desalination plant include source water intake system, pretreatment facilities and high pressure feed pumps, RO membrane trains, energy recovery and desalinated water conditioning system. The intake system may be the open surface water intake or series of seawater beach wells. The pre-treatment system may be the screening, chemical conditioning, sedimentation or filtration that totally depends on the used quality water further the filtered water is conveyed by transfer pump from filtrate water storage tank through cartridge filter and into the suction pipe of high-pressure RO feed pumps. The cartridge filters are designed in such a manner that can retain 1 to 20 microns particles which remained in the source water after pre-treatment. The high pressure feed pumps are designed to deliver the source water to the RO membranes at pressure required for membrane separation of the fresh water from the salts. The actual required feed pressure is site-specific and is mainly determined by the source water salinity and the configuration of the RO system.
Aeration occupies a significant place in waste-water quality management and is an important factor in the purification of polluted water. Gas transfer is a physical phenomenon in which gas molecules are exchanged between a liquid and a gas at a gas-liquid interface.
This physical phenomenon of gas molecules exchanged between the liquid and gas at the liquid-gas interface may also be accompanied by biological, biochemical, biophysical and chemical action. These results are often the primary purpose of the gas transfer operation and methods of achieving the desired results may vary. Principal objectives of aeration, is to add or remove gases or volatile substances, to water, or both the operations are carried out simultaneously.
In the biological process, the aerators help to transfer the required amount of oxygen and also ensure sufficient mixing of oxygen, to aerators function to transfer the required oxygen and include sufficient mixing to maintain uniform dispersed oxygen throughout the basin and keep biological solids in suspension in aerobic basins and the activated sludge process. For high-rate organic loadings, the power required may be determined by oxygen transfer requirements rather than mixing.
Sedimentation
Plain sedimentation does not remove suspended fine clay particles, silt particles that have a size of 0.06mm needs 10hours to settle in 3m deep sedimentation tank, and 0.02mm particles need about 4 days to settle .
Water cannot be kept for such a long time as the settling period is too long, however in plain tanks a 2-hour detention time can be permitted and about 6 hours for ordinary tanks is permitted.
Apart from fine suspended matter, electrically colloidal particles are also present in water, that are in a continuous motion and never settle due to gravitational force.
Therefore, a process is required that can remove the fine clay particles and colloidal impurities easily, after long experience it has been found that such impurities can be removed by sedimentation with coagulation
Process of Sedimentation with Coagulation:
It has been found that when certain chemicals are added to water an insoluble, gelatinous, flocculent precipitation is formed. This gelatinous precipitate during its formation and descent through the water absorb and entangle very fine suspended matter and colloidal impurities.
The gelatinous precipitate therefore has the property of removing fine and colloidal particles quickly and completely than by plain sedimentation. These coagulants further have the advantages of removing colour, odour and taste from the water. These coagulants if properly applied are harmless to the public.
Most commonly used coagulants:
- Aluminium sulphate [Al2, (SO4)3 18H2O]:
It is also called simply as alum. Alum which is available in market, is dirty grey solid in the form of lumps containing about 17% aluminium sulphate. This is the chemical coagulant which is widely used in water treatment plants. Alum reacts in water in the presence of alkalinity; if natural alkalinity is not present sufficient lime is added.
The following chemical reactions take place with the various types of alkalinity.
Al2(SO4)3 .18H2O+3Na2CO3-------> 2Al(OH)3+3Na2SO4+2CO2+15H2O
Al2(SO4)3 18H2O +3Ca(OH)2------>2Al(OH)3 +3CaSO4+18H2O
Al2(SO4)3 18H2O+3CA(HCO3)2------->2Al(OH)3+2CaSO4+6CO2+18H2O
The insoluble and colloidal aluminium hydroxide [Al(OH)3] forms the floe which removes the fine suspended and colloidal impurities. For best results the pH value of water should be between 6.5 and 8.5. The dose of alum should be 0.03 to 0.13 gm/litre depending on the turbidity of water
- Sodium Aluminate [Na2Al2O3]:
This is an alkaline compound. The best grade it contains Al2O3, 55%; Na2O3, 34%; Na2CO3 4.5%; Na (OH), 6.3%. This can be used for treatment very easily in the water having no alkalinity. It reacts very quickly and forms the precipitate of aluminium hydroxide.
Its chemical equations are as follows:
Na2Al2O3+CaSO4---->CaAl2O3+Na2SO4
Na2Al2O3+CaCl2 ----> CaAl2O3 +2NaCl
Na2Al2O3+Ca(HCO3)2 -----> CaAl2O3+Na2CO3+CO2+H2O
- Ferric Coagulants:
Generally ferric chloride (FeCI3), ferric sulphate [Fe2(SO4)3] or the mixture of both is used for coagulation purpose.
The various chemical reactions which take place are as follows:
2FeCl3+3Ca(OH)2---> 2Fe(OH)3+3CaCl2
Fe2(SO4)3+3Ca(OH)2 ------>2Fe(OH)3+3CaSO4
- Chlorinated Copperas
It is a mixture of ferric chloride and ferric sulphate prepared by adding chlorine to a solution of ferrous sulphate in the ratio of 1 part chlorine to 7.9 parts copperas.
6FeSO4+3Cl2----->2Fe(SO4)3+2FeCl3
1.4.1 Disinfection of water by chloramines, bleaching powder, chlorine and ozone.
Water disinfection involves the deactivation, killing and removal of pathogenic microorganisms, when microorganisms are deactivated their growth and reproduction is terminated. The presence of these organisms in drinking water causes illness and serious health problems. Sterilization is a process related to disinfection. However, during the sterilization process all present microorganisms are killed, both harmful and harmless microorganisms.
One of the commonly used disinfectants is Chlorine, it is known to be very effective in deactivating the pathogenic microorganisms, Chlorine is easily applicable, measured and controlled, they are relatively cheap and very persistent in nature. Chlorine has been used for applications, such as the deactivation of pathogenic microorganisms in waste water, drinking water and swimming pools.
The mode of action is that chlorine kills bacteria and viruses by breaking the chemical bonds in their molecules Chlorine kills pathogens such as bacteria and viruses by breaking the chemical bonds in their molecules. Chlorine can exchange atoms with other, like enzymes in bacteria and other cells. When the enzymes come in contact with chlorine, hydrogen atoms are replaced by chlorine in the molecule, resulting in the change in the shape of the compound and they may also fall apart, as the enzymes loose their activity the bacteria or the cell dies.
Bleaching powder (CaOCl2) can also be used as a disinfectant. It is produced by passing chlorine through calcium hydroxide (CaOH). The powder being in solid form makes it easier to be used in medical areas, when bleaching powder dissolves, it reacts with water to form underchloric acid (HOCl) and hypochlorite ions (OCl-). Bleach consists of chlorine gas dissolved in an alkali-solution, such as sodium hydroxide (NaOH). When chlorine is dissolved in an alkalic solution, hypochlorite ions (OCl-) are formed during an auto redox reaction. Sodium hypochlorite is formed when chlorine reacts with sodium hydroxide and is stable and a good disinfectant, however bleach cannot be combined either acids as they form unstable products.
Chloramines are also called as secondary disinfectants,, as ammonia is first added to chlorine present in water, ammonia is added after chlorine, because this causes CT values to be lower than when ammonia is added primarily.
Comparatively chloramines are effective as Chlorine as disinfectant are effective in deactivating the pathogenic microorganisms., the mechanism of reacton however is slower, like Chlorine they are also oxidators, their mode of action involves penetration into the bacterial cell wall and blocking its metabolism, among chloramines the most effective disinfectant is Monochloramine, as it directly reacts with the amino acid of the bacterial DNA, During the deactivation process the chloramines are capable of destroying the shell that protect the virus. The pH value does not interfere with the effectiveness of chloramines.
Ozone are also potent germicides and powerful oxidisers apart from being good disinfectants, ozone is an unstable gas and can kill bacteria and viruses effectively, they are powerful oxidisers, with no build-up immunity or toxic residues. Ozone is most effective disinfectant compared to chlorine and other commonly used disinfectants. Ozone is short-lived, they return to their normal form when it breaks. When ozone destroys bacteria or any organic compound, the free atoms liberated attack any foreign particle that are present in air or water, however during this process ozone does not produce any toxic compounds unlike chlorine compounds.For the decontamination of water or air, ozone is considered the most effective and a safe disinfectant, at the right levels and safety standard they can be used effectively in hospitals, industries and homes. As ozone is involved in both removing the microorganism and disinfection of microbes, they are also effective in waste water treatment, medical fertilisers. Food processes etc.
Reference:
1. Engineering Chemistry, Jain and Jain, Dhanpat Rai & Co
2. Engineering Chemistry, M. Subha Ramesh, Dr. S. Vairan-Ed.-IInd Wiley
3. Instrumental Methods of chemical analysis, Chatwal and Anand,Himalaya Pub House
4. Industrial Chemistry, B.K.Sharma,Goyal
5. Chemistry for Engineers, Rajesh Agnihotri, Wiley
6. Fundamentals of Engineering Chemistry, S.K.Singh, New Age Int.
7. Engineering Chemistry (NPTEL Web book), B. L. Tembe, Kamaluddin & M. S. Krishnan.
8. A text book of Engineering Chemistry, S.S. Dara, S Umare, S Chand
9. A text book of Engineering Chemistry, Shashi Chawala, Dhanpat Rai & Co
10. A text book of Experiments and Calculations in Engineering Chemistry, S.S. Dara. S Chand