Unit- 5
Metal, Alloys & Ceramics
- Metallic materials are substances that exist in combination, they are combined with metallic elements such as titanium, iron, aluminium and gold, these metallic materials are inorganic substances, the materials may also contain small amounts of elements that are non-metallic like carbon, nitrogen and oxygen. The usage of metals in the pure form is rare, but are usually mixed with other elements to form an alloy.
Cast iron, cast iron are alloys that comprise of 2-4 % of carbons along with different amounts of manganese and silicon and also traces of impurities such as phosphorus and sulphur. The cast iron is obtained by reducing iron ore in a blast furnace, the liquid iron is poured and hardened into slabs or lumps called pigs, subsequently these pigs are remelted along with the scrap and alloying elements in cupola furnaces and recast into molds, for producing a range of products.
Cast iron are brittle in nature except for malleable cast irons, they have low melting point, good machinability, good fluidity, castability and show good resistance to deformation and are wear resistant. Cast irons have become a very good engineering material with a wide range of applications.
Applications:
- It is used in making pipes, to carry suitable fluids
- It is used in making different machines
- It is used in making automotive parts
- It is used in making pots pans and utensils
- It is used in making anchor for ships.
Steel: Steel, alloy of iron and carbon in which the carbon content ranges up to 2 percent (with a higher carbon content, the material is defined as cast iron). It is the most extensively used material for building industries and infrastructure. Stell gains immense popularity due to its relatively low cost of forming, processing and making it. The raw material iron ore and scrap are always in abundance and has its un parallel range of mechanical properties.
Applications:
- It is used to fabricate everything from sewing needles to oil tankers,
- Steel's versatility has made it the most widely used—and most recycled—metal material on Earth. In addition,
- Its high strength and relatively low production cost make it suitable for use in countless applications, including in railways, boats, bridges, cooking utensils, packaging, and electrical transformers.
Wrought Iron
- Wrought iron, one of the two forms in which iron is obtained by smelting; the other is cast iron (q.v.). The properties of wrought iron include, they are soft, fibrous and ductile. Wrought iron is formed from a semi fused mass of relatively pure iron globules partially surrounded by slag. It usually contains less than 0.1 percent carbon and 1 or 2 percent slag. It can be heated and reheated and worked into various shapes.
Applications of wrought iron:
- Decorative items such as railings, outdoor stairs, fences and gates
- Nuts and bolts
- Handrails.
5.1.2 Alloys: definition, purposes of making alloys.
Alloy, are metallic substances that consist of two or more elements, either in a solution or compound, the constituents of alloys are metal themselves, although carbon which is a nonmetal is an essential constituent of steel.
Alloys are usually formed by melting the mixture of ingredients, alloys were discovered in ancient times, like brass (copper and zinc) Alloys are usually produced and bronze (tin and copper) were especially important.in todays world the alloy steels are the most important ones. Broadly defined as steels containing significant amounts of elements other than iron and carbon.
Purpose of making alloys:
- Enhance the hardness of a metal: alloys are usually harder than all its components, on the other hand pure metals are softer, therefore the hardness of the metal can be improved by alloying with another metal or non metal.
- Lower the melting point: In general Pure metals have a high melting point. Therefore, the pure metals are alloyed with either a metal or non metal to lower its melting point, this property makes metals feasible and is useful in making alloys called solders.
- Increase tensile strength: Alloy formation increases the tensile strength of the parent metal.
- Enhance corrosion resistance: compared to pure metals alloys are resistant to corrosion, pure metals can be corroded by the surrounding moisture and atmosphere, thus alloying these metals helps to increase the inertness of the metal, and thereby increase resistance to corrosion.
- Modify colour: Colour pigments present in metals or nonmetals are used to alloy it with pure metal to obtain the desired colour
- Provide better castability: One of the most essential requirements of getting good castings is the expansion of the metal on solidification. Pure molten metals undergo contraction on solidification. Metals need to be alloyed to obtain good castings.
Ceramic: when materials such as clay are made into suitable shapes and fired to very high temperatures, the resulting material formed is a ceramic. Ceramics are hard brittle, corrosion resistant and heat resistant.
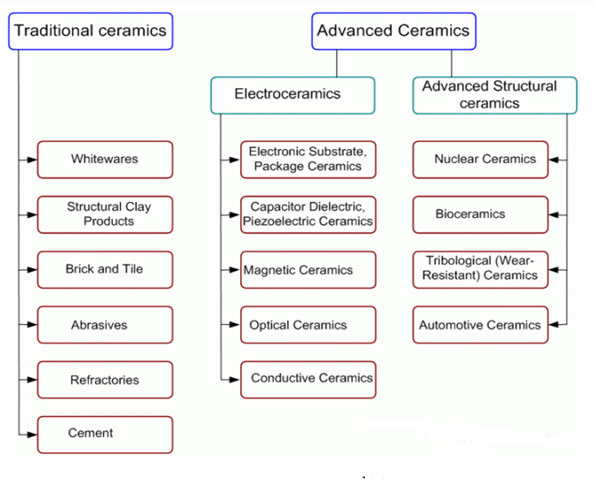
The classification of Ceramics based on application
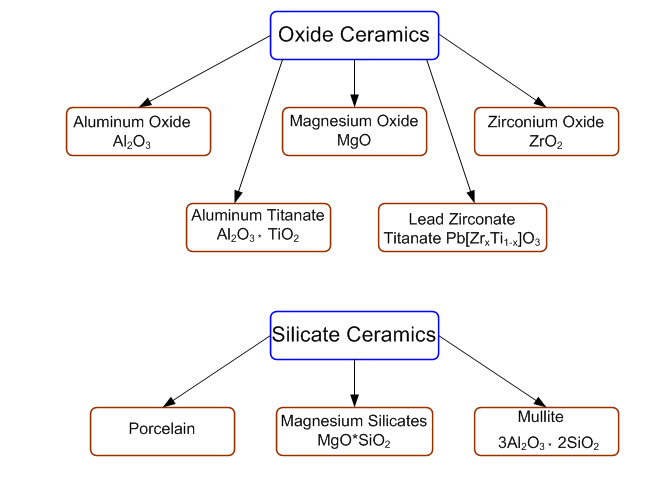
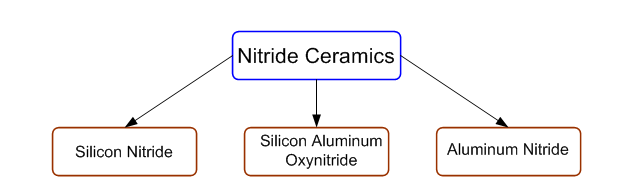
Classification based on composition base
General Properties of Ceramics:
- They are brittle and hard in nature they may be amorphous and glossy.
- Ceramics are bonded by covalent and ionic bonds, due to which no free electrons are present in its structure, that makes them thermal and electrical insulators.
- They deform elasticity at low temperatures, but can experience viscous flow at certain stress conditions.
5.2.1 Glass: general properties, general method of manufacture of glass, types of glasses: soft, hard, borosilicate, optical, laminated and safety glass.
Glass, is a solid inorganic material, that may be translucent or transparent, they are obtained by cooling molten images like silica sand rapidly to avoid the formation of crystals.
Properties of glass
- Hardness and Brittleness
It is a hard material as it has great impact resistance against applied load. However, at the same time, it is a brittle material as its breaks immediately when subjected to load
- Weather Resistance
It is weather resistant as it can withstand the backlash of rain, sun and wind. It can absorb, reflect and refract light as it enables us to control and manipulate natural light to influence our daily activities and regulate our mental and physical health.
It has great dimensional stability as it has low thermal expansion value. (i.e., its change in volume with respect to temperature change as compared to other materials is very low.)
- Insulation
It is an excellent insulator against heat, electricity and electromagnetic radiation because of its good insulating response against visible light transmission.
Certain special type of glass has high resistance against ultra-violet, infrared and x-ray transmission. It has an excellent resistance against sound transmission provided used with appropriate thickness.
- Chemical Resistance
It can withstand the effect of the chemical reaction under different environment conditions or acidic effects.
It has excellent resistance to most chemicals including solutions of inorganic alkalis and acids such as ammonia and sulfuric acid.
- Colour and Shape Varieties
It can be blown, drawn and pressed to any colour, shape, and variety and is available in the market depending upon their use, dimensional requirements, and safety requirement.
- Transparency
The transparency is one such property of glass which creates a visual connect with the outside world. With the advent of technology, clear glass can also be altered, making it opaque.
- Property Modification
It is also possible to change some of its properties to suit different purposes. The major surface modification processes are listed below, and their names itself suggest the different properties of glass to which it can be modified depending upon their use in the building.
Manufacture of Glass
The manufacturing process of glass consists of four major operations: (1) Melting, (2) Shaping, (3) Annealing, (4) Finishing.
Each operation is being discussed briefly as follows:
1. Melting. This is the first process in the preparation of glass, the ingredients are called batch materials (Sand and soda) are taken and mixed in the right proportion, and heated to fusion in a furnace. The two most commonly used furnaces are: (i) Pot furnace and (ii) Tank furnace.
i. Pot Furnace. The pots in this furnace may be closed or open, the charge is fused in fire clay pots. The pots may be closed, when the glass needs to be protected from the products of combustion.
Once the batch material is put in the pots, they are placed in the furnace in a circular manner as shown in the figure, they are then heated by burning producer gas around them. When the fusion in the pots is completed, the fused plastic mass is removed from the furnace and further it is shaped. As the charge in the furnace remains protected from the products of combustion, the pot furnace is used to produce high quality glass.
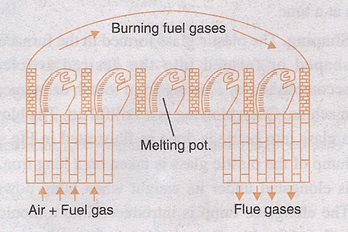
Pot furnace.
(ii) Tank Furnace. As the name suggest, it is made up of a large rectangular tank built with fire clay blocks. The batch materials are fed into the tank and fuel gas is used in the furnace.
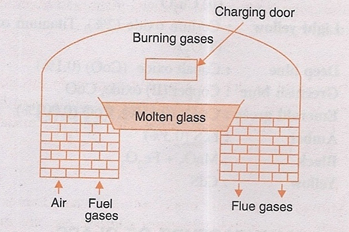
Tank furnace.
The charge is heated at 1400°- 1500°C for 10-12 hours.
The chemical reactions involved in both the furnaces are:
Na2CO3 + SiO2 → Na2SiO3 + CO2
2Na2SO4 + 2SiO2 → 2Na2SiO3 + O2 + 2SO2
CaCO3 + SiO2 → CaSiO3 + CO2
At 1400°C silica also in silicates of calcium and sodium
Na2SiO3 + CaSiO3 + 4SiO2 → Na2SiO3.CaSiO3 .4SiO2
Glass
During melting a lot of froth is formed due to the evolution of gases like CO2, SO2, O2, etc. when the frothing reduces, the temperature is increased and allowed to stand for some time, this helps in the formation of a uniform mass free of gas bubbles, bits of undissolved material or batch stones. This process is called refining. Tank furnace is a continuous process and usually employed for the production of large quantities of only one variety of glass at a time.
2. Shaping. The plastic glass formed from the furnace are further shaped into desired articles. The glass is then subjected to blowing, the steps are shown in the figure, A lump of the fused plastic glass is taken in a long iron pipe, due to the weight of the lump the pipe begins to elongate and hang downwards, the elongated lump is then introduced to a mould, and then inflated by blowing air into it, after the desired shape from the mould is obtained, they are cooled and removed from the mould.
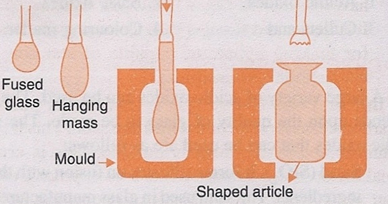
Shaping of glass lump.
3. Annealing. Is a process of slowly cooling the newly formed article, the process is slow, if carried out quickly the article becomes brittle due to internal strain It is a process of cooling slowly the newly shaped articles. Annealing therefore helps the article to cool slowly and allows the molecules to arrange themselves in such a way that there is no internal strain. A 50-60 feet long tunnel like oven called lehr is used for Annealing, at one end of the tunnel the temperature is 500-600OC, this temperature is gradually reduced along the length of the tunnel, the temperature at the other end of the tunnel is about the room temperature, the mass is placed initially on the hotter end slowly cooled along this tunnel as it moves along a moving belt. It takes a few hours for the articles to move along through the tunnel. Some high-quality glasses require long annealing.
4. Finishing. The articles obtained from the lehr are subjected to a number of operations such as cleaning, polishing, grinding, rounding edges, etc., for bringing them to a useable form.
Types of Glasses
Hard-glass: is an acronym for borosilicate glass, like Pyrex, Lab glass is generally made from hard glass.
Soft-glass: is an acronym for soda-lime glass, or a higher expansion type glass, Bottles are generally made from “soft-glass,” or soda-lime glass.
Safety glass: also called as shatterproof glass, it is made by placing a sheet of plastic such as celluloid between sheets of glass, the special feature in this glass is in cases of breakage the broken piece sticks to the plastic and does not fly off, it is mostly used in automobiles and bullet proof screens.
Laminated glass: It is also called bullet proof glass, here several layers of safety glass are bound together with a transparent adhesive, the larger the number of layers the greater is the strength of the glass, it is stronger than safety glass, used mainly in aeroplanes and window shield of cars.
Optical glass: is softer than any other glass, they are clear and transparent
Potassium and lead silicates are used to make optical glass, they are also called flint glass, the main use of flint glass is in the manufacture of lenses, prisms and optical instruments.
Borosilicate glasses: They are highly heat resistant, in ordinary glass silica is the main constituent, in a borosilicate glass, some of the silica is replaced by boron oxide, boron oxide expands very slowly on heating, thus these glasses do not crack while strong heating. They are also called Pyrex glasses
It has high melting point and resistant to various chemicals, the Pyrex glass is used in laboratory ware and oven ware.
Reference:
1. Engineering Chemistry, Jain and Jain, Dhanpat Rai & Co
2. Engineering Chemistry, M. Subha Ramesh, Dr. S. Vairan-Ed.-IInd Wiley
3. Instrumental Methods of chemical analysis, Chatwal and Anand,Himalaya Pub House
4. Industrial Chemistry, B.K.Sharma,Goyal
5. Chemistry for Engineers, Rajesh Agnihotri, Wiley
6. Fundamentals of Engineering Chemistry, S.K.Singh, New Age Int.
7. Engineering Chemistry (NPTEL Web book), B. L. Tembe, Kamaluddin & M. S. Krishnan.
8. A text book of Engineering Chemistry, S.S. Dara, S S Umare, S Chand
9. A text book of Engineering Chemistry, Shashi Chawala, Dhanpat Rai & Co
10. A text book of Experiments and Calculations in Engineering Chemistry, S.S. Dara. S Chand