Unit-6
Fuels
Definition: - A fuel is a substance that contains carbon and hydrogen undergoes combustion in presence of oxygen to gives large amount of energy.
 Fuel + O2 CO2 + H2O + Energy
Fuel + O2 CO2 + H2O + Energy
Fuels:-
A fuel is the substance which on combustion produces a large amount of heart.
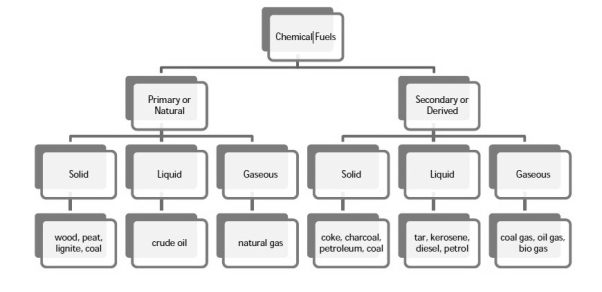
6.1.1 Classification
Chemical fuels are classified on the basis of occurrence into :-
- Primary ( natural )
- Secondary (derived ) types / manmade .
The secondary fuels are obtained from primary fuel by processing or they are manmade. e.g.:- charcoal is obtained from wood by partial combustion of wood , ethyl alcohol is obtained by fermentation of carbohydrates .
Both the primary and secondary fuels are further classified on the basis of physical state into solid liquid and gaseous fuels.
Extra definitions:-
1] Fuels:-
Fuels are defined as materials that create usable energy through chemical nuclear or electrochemical reaction.
2] Chemical fuel:-
The fossil fuels, wood vegetal oils etc. . which produce heat on burning are known as chemical fuels.
6.1.2 Characteristics of good fuel
Biodiesel can be used as a good fuel for diesel engine but generally it is used as its 20 percent mixture with diesel.
- Biodiesel is cheaper
- It has high c.v of about 40 kg / gm
- It is regenerative and environmentally friendly.
- It does not give out particulate and CO pollutants.
- It has certain extent of lubricity
- It uses provided good market to vegetable oils and reduces our dependence on biodiesel on foreign countries, saving currency.
- It is clean to use biodiesel in diesel engine.
6.1.3 Comparison between solid, liquid and gaseous fuel
Comparison between solid, liquid, and gaseous fuels.
SOLID FUELS | LIQUID FUELS | GASEOUS FUELS |
|
|
|
|
|
|
6.1.4 Calorific value (gross and net)
Calorific value: Efficiency of combustible fuels can be measured in terms of their calorific value. It is the heat evolved on combustion of a unit quantity of fuel.
Types of calorific value (Higher and lower)
Higher Calorific Value:
- Gross calorific value of a fuel can be defined as the total amount of heat obtained on complete combustion of unit mass of a solid or liquid fuel or unit volume of a gaseous fuel ( S T P ) and on cooling the products of combustion to 15 degree c. The gross calorific value is also called as higher calorific value.
- The G.V.C is of only theoretical importance because in actual practice we do not have any provision of cooling the products of combustion during combustion of fuel in an engine, furnace or any other fuel burning device.
Lower Calorific Value:
- Net calorific value is defined as the amount of heat obtained practically on complete combustion of unit mass of solid or liquid fuel or unit mass of solid or liquid fuel or unit volume of a gaseous fuel at step and the products of combustion are allowed to escape with some heat N.C.V is also called as lower calorific value.
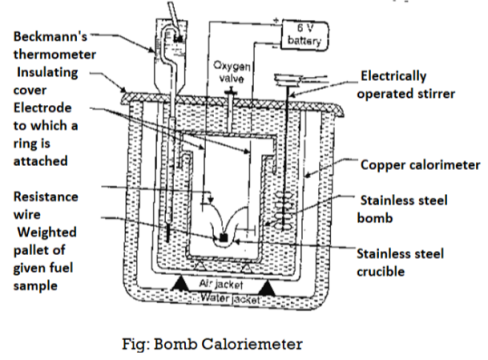
Bomb Calorimeter
The gross calorific value of solid fuels and liquid fuels can be determined by bomb calorimeter. (If the liquid is volatile, then it is filled in a polythene capsule of negligible mass then used in experiment.
Construction: A bomb colorimeter consists of
- Bomb pot
- Calorie meter
- Water and air jackets
- Accessories
- Pellet press
- Oxygen cylinder
Bomb pot :-
It is a cylindrical strong stainless-steel pot having a lid . The lid can be fitted air. Tight to bomb pot by screwing.
- There are two type of electrodes fitted through lid and there is an oxygen inlet valve as its center.
- One of these electrodes is provided with a ring to hold the crucible containing fuel .there is thin resistance wire tied to the electrodes in loop form and the loop touches the fuel.
- The weighed fuel is burnt in the bomb pot in the presence of high-pressure oxygen.
Calorimeter :-
- There is a stainless steel or copper calorimeter in which the bomb pot is kept .it contains a known volume of water and the water is kept circulating around the bomb pot with the help of a stirrer.
- A Beckman thermometer or digital thermometer is kept in the water of calorimeter, which can record the rise in temperature of welter due to absorb in a heat generated .
- There are insulator stands between calorimeter and water jacket.
Accessories :-
- There is a pellet press to convert the powder of solid fuel to pellet form .for a liquid fuel a capsule of negligible weight can be used.
- There is an oxygen cylinder with pressure gauge to fill oxygen in the bomb pot at the pressure of nearly 25 kg / cm².
- There is also a D.C battery of a about 6 volts to start combustion of fuel.
Working :-
- Weigh the pellet of solid fuel or liquid capsule and keep it in the crucible .keep the crucible in the ring of the electrode .keep the resistance wire touching to the fuel.
- Add about 10 ml of distilled water at the bottom of bomb pot and fix the lid tightly to bomb by screwing.
- Fill the bomb with oxygen at the pressure about 25 kg / cm².
- Place the bomb in calorimeter add known volume of water in the calorimeter so that the bomb gets immersed in the water.
- Place the calorimeter in the water jacket over the plastic studs .keep the thermometer and stirrer in the water of calorimeter.
- Put the plastic cover on the and make electrical connections from battery to electrodes.
- Operate the stirrer for s minutes and note the initial temperature of water ( t1° c ).
- Pass the current for about 5 – 10 seconds to heat the wire so that the fuel catches fire. If the fuel contains S and N elements, they get converted to SO3 and N2O5 .these gases get dissolved in the distilled water in bomb to form H2SO4 and HNO3 ( along with liberation little heat ).
- Note the maximum temperature reached .after that note the rate of fall of temperature per minute and the time taken for reaching to initial temp. Are noted.
- Open the bomb pot and wash the contents at its bottom into a beaker to find out the amount of H2SO4 and HNO3 formed.
Boy’s Calorimeter:
Principle:-
The gaseous fuel is burnt at a known constant rate in the calorimeter under such conditions that entire amount of heat produced is absorbed by water.
This calorimeter is used to measure calorific value of gaseous fuels and highly volatile fuels.
- Gas burner
- Combustion chamber (chimney)
- Thermometers
- Insulating cover
- Gas burner:-
- There is a gas burner in which a known volume of gas is burnt at a known pressure. The gas is burnt at the rate of 3 – 4 lit. Per minute.
3. Combustion chamber (chimney) :-
Around the burner there Is a combustion chamber which has a copper tubing coiled inside as well as outside of it water enters from top of the outer coils , moves to bottom of chimney and then goes up through the inner coil to the exit at top.
4. Thermometer :-
There are two thermometer to measure temperatures of inlet water and outlet water.
5. Insulating cover :-
The assembly is covered with by an insulator to detach combustion chamber from atmosphere. There are three holes for exhaust gas, water inlet and condensed steam.
Working :-
- Start burning the gas at suitable pressure and adjust the rate of water flow such that the temperature of outgoing water remains constant.
- Burn the gas for 5 – 10 minutes to have the steady temperatures in and around the combustion chamber.
- After the steady (temperature in and around the combustion chamber ) conditions of outgoing.
A) Volume of gas burnt at given temperature and pressure in certain time period.
B) Quantity of water passed through coil during this period.
C) Mass of water condensed from product gas during the period.
D) The steady rise in temperature of water ( t2 – t1 )
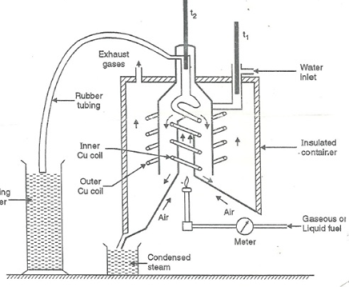
Fig: Boy’s gas calorimeter
Calculations :-
- First convert the volume of gas burnt to volume of gas at STP .let this STP volume be V
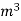 .
. - Let W = mass of cooling water used in the period of observation in Kg
- Let m= mass of water condensate in kg
- L= G.C.V OF THE FUEL
Heat produced by combustion of fuel = heat absorbed by cooling water
( assuming no heat loss in the steady state conditions )
Vl = w ( t2 – t1 )
L = 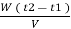 Kcal /
Kcal / 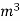
The mass of condensate water per m of gas will be m/v kg /m .
If this water had left as steam in product gases it would have taken away heat.
= 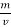 *587 kcal /
*587 kcal / 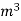
Therefore ,
NCV = GCV - 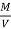 *587
*587
NCV = 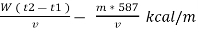
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Numerical problems on Bomb and Boy’s calorimeter
A 1.000 g sample of octane (C8H18) is burned in a bomb calorimeter containing 1200 grams of water at an initial temperature of 25.00ºC. After the reaction, the final temperature of the water is 33.20ºC. The heat capacity of the calorimeter (also known as the “calorimeter constant”) is 837 J/ºC. The specific heat of water is 4.184 J/g ºC. Calculate the heat of combustion of octane in kJ/mol.
Since this is a combustion reaction, heat flows from the system to the surroundings- thus, it is exothermic. The heat released by the reaction will be absorbed by two things: (a) the water in the calorimeter and (b) the calorimeter itself.
- Calculate the heat absorbed by the water (qwater)
m = 1200 grams
Cwater = 4.184 J/gºC
∆T = 33.20 – 25.00 = 8.20ºC
Qwater = (m)(c)(∆T), so
Qwater = (1200 g)( .4 184)( .8 20 C)
=41170.56 J
= 41.2 kJ ⋅
b. Calculate the heat absorbed by the calorimeter (qcal)
The temperature change of the calorimeter is the same as the temperature change for water. In this step, however, we must use the heat capacity of the calorimeter, which is already known. When using heat capacity, the mass of the calorimeter is not required for the calculation. (It’s already incorporated into the heat capacity).
Ccal = 837 J/ºC
∆T = 33.20 – 25.00 = 8.20ºC
Qcal = (Ccal)(∆T),
So,
Qcal = (837 )( .8 20 C) = 6863 J 4. = 6.86 kJ
6.2.1 Dulong’s formula for calorific value. (Numerical problems on calorific value.)
Petrol is a volatile flammable liquid, that consists of a mixture of hydrocarbons namely heptane, hexane and octane. They are mainly obtained from petroleum and used as a solvent and fuel for internal combustion engines. Petrol also contains additives such as antiknock compounds and corrosion inhibitors.
Elemental Composition of Petroleum
Although there is considerable variation between the ratios of organic molecules, the elemental composition of petroleum is well-defined:
- Carbon - 83 to 87%
- Hydrogen - 10 to 14%
- Nitrogen - 0.1 to 2%
- Oxygen - 0.05 to 1.5%
- Sulfur - 0.05 to 6.0%
- Metals - < 0.1%
The most common metals are iron, nickel, copper, and vanadium.
Classification:
The hydrocarbons in crude oil can generally be divided into four categories:
- Paraffins: These have a carbon ratio of 1:2 and form up to 15 to 60% of crude, the ratio 1:2 means that they contain twice the amount as they do for carbon. They are compounds that are never circular they are either in straight or branched chains. The shorter the paraffins are, the lighter the crude is.
- Napthenes: they are also called as cyclic paraffins, they possess cyclic compounds and have the carbon and hydrogen ratio of 1:2. They form about 30-60% of crude. They are generally more viscous and higher in density than paraffins.
- Aromatics: Burning aromatics form soot hence they are undesirable and form around 3 to 30% of crude. They show much less hydrogen in comparison to carbons that are present in paraffins. They have a much less hydrogen in comparison to carbon than is found in paraffins. They are also more viscous. They may exist in a solid or semi-solid when an equivalent paraffin would be a viscous liquid under the same conditions.
- Asphaltic: The hydrogen carbon ratio is1:1 and form about 6% of the crude, they are dense in nature and undesirable, but their 'stickiness' makes them excellent for use in road construction.
Origin
- They are mainly formed the remains of dead plants and animals.
- When animals and plants usually die, they settle and sink on the seabed.
- Many millions of years ago this wildlife and vegetation decomposed and they mixed with silt and soil.
- Some specific Bacteria act on this decomposed organic matter and caused few chemical changes
- The matter could not be decomposed completely, s there is no sufficient oxygen present in the sea, however matter consisting of largely carbon and hydrogen was left behind.
- The decomposed matter remained on the sea bed and eventually was covered with multiple layers of sand and silt.
- After millions of years, due to high temperature and pressure finally the organic matter decomposed completely and formed oil
Refining of crude oil
In an oil refinery the crude oil is converted into valuable goods such as petroleum, naphtha, gasoline liquified gas, kerosine etc. Oil refineries are usually huge, vast industrial facilities with extensive pipelines running throughout, holding fluid streams in between.
- Many substances are present in petroleum as a mixture, they include lubricating oil, paraffin wax, diesel, gas, wax etc.
- Therefore, it becomes important to separate these substances and use them separately for different purposes, hence they are refined and the process of separation various constituents of petroleum is called petroleum refining.
- It involves a three-step process and usually performed in oil refineries.
- The first step is distillation process, that separates the crude oil from various components. The lighter constituents rise up as vapour or remain as a liquid while the heavier constituents remain at the bottom.
- In the next step the heavier constituents are converted into gas, gasoline and diesel.
- The last step is to remove the impurities in the constituents and obtain the products in pure form. The most common method of separating components into their constituents is by fractional distillation when due to boiling differences in temperature the crude oil heat up, spray and then condense the vapour.
- New methods, in a method called conversion, use Chemical processing on certain fractions to produce others. For example, chemical processing may split lengthier chains into shorter chains. This allows a refinery to convert diesel fuel into gasoline, depending on the gasoline demand.
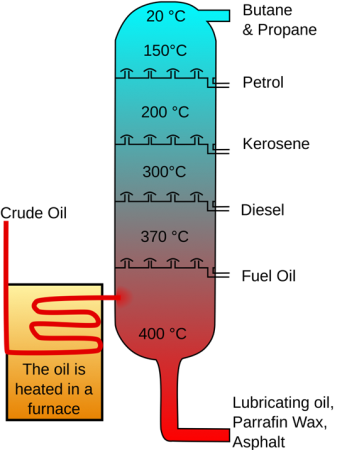
Crude Oil Distillation
In industries, the process of refining is usually called the downstream sector, and the raw crude oil output is called the downstream sector the word downstream is synonymous with the idea of sending oil down the supply chain of a commodity to an oil refinery to be refined into petrol. The downstream also indicates the sale of petrol and its products to other companies and government and private sectors.
6.3.1 Biodiesel: introduction, preparation, advantages and disadvantages.
The common plants used for biodiesel production is Soybean and oil palm. Microorganisms such as algae and bacteria also form sources of biodiesel, they seem promising but are economically difficult to develop Few species of algae have up to 40% lipids by weight that can be converted to synthetic petroleum or biodiesel.it is estimated that few algae and cyanobacteria yield between 10 and 100 times more fuel per unit area than plant-based biofuels.
Is a fuel where the chemical energy stored in the fuel is converted into mechanical energy, any internal combustion engine, where air is compressed to very high temperatures to ignite the diesel fuel injected into the cylinder. The piston is actuated by combustion and expansion, the mechanical energy can to use to drive freight expansion, The tractors and marine vessels. A limited number of automobiles also are diesel-powered, as are some electric-power generator sets.
Preparation
The preparation of the biodiesel is a transesterification reaction where the triglycerides are converted into simpler methyl esters of the fatty acids (the biodiesel). Biodiesel is produced from tallow, vegetable oil or animal fat, and waste oils. There are three stages of this transformation of oil and fats to biodiesel.
a) Transesterification of the oil in which it is base-catalysed.
b) The direct acid-catalysed transesterification
c) Finally, conversion of oil to fatty acid and then the formation of biodiesel.
The production of biodiesel involves a chemical reaction. This chemical reaction is known as transesterification.
Transesterification is the chemical process, which converts natural fats and oils into Fatty Acid Methyl Esters (FAME) or Biodiesel. Some of the major sources of suitable oil (to make biodiesel) come from crops like palm, soybean or rapeseed.
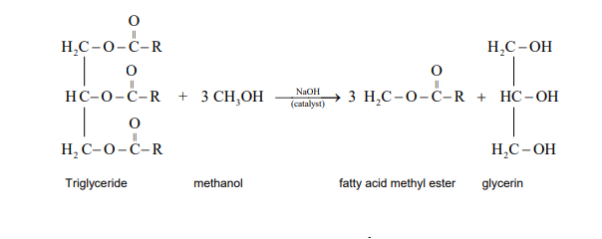
Advantages:
- As Biodiesel is a renewable energy source, they will not vanish away like many petroleum products, since they are made from vegetable oils they can be produced whenever necessary and they cause less pollution.
- One of the distinct advantages of using biodiesel is they can be used in existing diesel engines with no or very less modifications and can form the primary transport energy source.
- Usage of biodiesel helps to reduce, burning of fossil fuels as they cause pollution and global warming.
- The biofuel serves as an alternate form of fuel, thus reduce our dependence on foreign supplies of oil, as biofuels are produced from domestic energy crops.
- 305 fuel economy is achieved by vehicles that run on biodiesel than the one that use petrol-based engines, which means it makes fewer trips to gas stations and runs more miles per gallon.
Disadvantages:
- Biodiesels are produced from crops, most of the crops are treated with fertilizers that can affect the environment and cause pollution, excess use of fertilizers cause soil erosion.
- As they are produced from crops and animal fat, their demand may rise leading to a shortage of food crisis
- Biodiesel are not suitable in low temperatures, they form gel under low temperatures, which depends on the oil or fat used to make it
- The engine efficiency increases with the use of biodiesel, it can cause considerable damage to the rubber house of some engines.
Reference:
1. Engineering Chemistry, Jain and Jain, Dhanpat Rai & Co
2. Engineering Chemistry, M. Subha Ramesh, Dr. S. Vairan-Ed.-IInd Wiley
3. Instrumental Methods of chemical analysis, Chatwal and Anand,Himalaya Pub House
4. Industrial Chemistry, B.K.Sharma,Goyal
5. Chemistry for Engineers, Rajesh Agnihotri, Wiley
6. Fundamentals of Engineering Chemistry, S.K.Singh, New Age Int.
7. Engineering Chemistry (NPTEL Web book), B. L. Tembe, Kamaluddin & M. S. Krishnan.
8. A text book of Engineering Chemistry, S.S. Dara, S S Umare, S Chand
9. A text book of Engineering Chemistry, Shashi Chawala, Dhanpat Rai & Co
10. A text book of Experiments and Calculations in Engineering Chemistry, S.S. Dara. S Chand