Unit – 7
Polymers
The process in which the simpler molecules combine together to form very large molecule having high molecular weight is known as polymerization. The molecule is known as polymer. The different ways of doing polymerization are –
By opening a double bond
Ex:
NCH2 = CH2 (CH2 – CH2)n
Ethane polythene
NCH2=CH (CH2 – CH) n
Cl Cl
Vinyl chloride polyvinyl chloride
7.1.1 Types of polymerization (no mechanism)
Polymers can be synthesized by the following polymerization processes:
- Addition polymerization or chain polymerization
- Condensation or step or step growth polymerization
- Copolymerisation
Addition polymerization:
The addition polymerization is the process in which the linking together of monomer molecules by a chain reaction is observed. Polymer synthesized by addition polymerization has the same empirical formula as that of monomer. No molecule is evolved during polymerisation and the polymer is an exact multiple of the original monomeric molecule.
Condensation polymerization:
An intermolecular reaction involving two different bifunctional reactants with affinity for each other and taking place through repeated condensation reaction is known as condensation polymerization.
Copolymerisation:
Addition polymerisation involving a mixture of two or more suitable or compatible monomers gives a copolymer and the process is known as copolymerization. A reaction in which a mixture of two or more monomers is allowed to undergo polymerisation is known as copolymerization. The polymer is known as copolymer.
E.g.: Copolymerisation of styrene and methyl methacrylate
7.1.2 Degree of polymerization (DP)
For a given polymer, the degree of polymerization is defined as the number of repeated units present in the polymer molecule. The term is also sometimes used to express the monomer units present in an average polymer molecule. Therefore, this becomes applicable to the repeated units of single type of monomer.
It is usually denoted as ‘n’ in the generalized formula
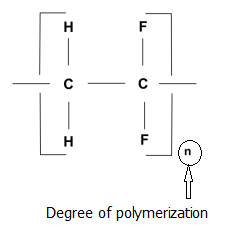
- [ M] n; where M is the repeating unit.
A polymer sample mainly contains the distribution of chains that possess different degree of polymerization. Therefore, while determining DP an average value is taken. If a polymer molecule of known molecular weight is taken then the following formula can be used.
M= (DP) M0
M is the molecular weight of the polymer; DP is the degree of polymerization and the M0 is the formula weight of the repeating unit.
7.1.3 Numerical problems on degree of polymerization
Calculate the degree of polymerization of a sample of polyethylene [ (CH2-CH2) n], which has a molecular weight of 150,000 g/mol.
The molecular weight of a repeating unit, Mo= (12 x 2 + 1 x 4) g/ mol = 28 g/mol
DP = M/Mo
= 150,000 g/mol / 28 g/mol
= 5.35 x 103
The particular molecule contains 5.35 x 103 of repeat units.
When considering the molecular weight of a polymer for the above calculation, we usually take either the number average molecular weight (Mn) or weight average molecular weight (Mw).
7.1.4 Number average molecular weight (definitions and numerical problems
Molecular Weight:
Molecular weight is the average weight of the molecules that make up a polymer and gives an indication of the length of the polymer chains.
The polymerization process is subject to variation so there is no single chain length, there is actually a wide range of lengths, so when we discuss molecular weight, we really mean the average molecular weight of the material. This average is found by measuring samples of the material as it is produced.
Unless they have been purified, synthetic polymers have a distribution of molecular weights. Different methods of measuring the molecular weight yield different types of averages. Two of the most important are the number-average and the weight-average molecular weights. Suppose that you have a set of values {x1, x2, . . ., xn} for which you calculate an average value. If the probability of each value occurring are given by {P1, P2, . . ., Pn} then the average is given by the sum below:
∑i=0∞Pixi
Numerical problems on molecular weight:
a. To Find:
(a) The number-average molecular weight
(b) The degree of polymerization for the given polypropylene material b. Given:
Molecular Weight Range | xi | wi |
8,000–16,000 | 0.05 | 0.02 |
16,000–24,000 | 0.16 | 0.10 |
24,000–32,000 | 0.24 | 0.20 |
32,000–40,000 | 0.28 | 0.30 |
40,000–48,000 | 0.20 | 0.27 |
48,000–56,000 | 0.07 | 0.11 |
c. Assumptions:
The given data is accurate; the material (polypropylene) is pure.
Solution:
Number-average molecular weight:
Molecular Weight Range | Mean Mi | xi | xiMi |
8,000–16,000 | 12,000 | 0.05 | 600 |
16,000–24,000 | 20,000 | 0.16 | 3200 |
24,000–32,000 | 28,000 | 0.24 | 6720 |
32,000–40,000 | 36,000 | 0.28 | 10,080 |
40,000–48,000 | 44,000 | 0.20 | 8800 |
48,000–56,000 | 52,000 | 0.07 | 3640 |
Mn=xiMi
= 33,040 g/mol
Degree of polymerization:
For polypropylene, the repeat unit molecular weight,
m = 3(AC ) + 6(AH )
= (3)(12.01 g/mol) + (6)(1.008 g/mol)
= 42.08 g/mol
DP = Mn/ m
= 33,040 /42.08 g/mol
= 785
7.2.1 Definition
Synthetic or semi-synthetic polymers are plastics. Plastics used for industrial work come from petrochemicals. Plastic refers to its ability to deform without breaking. The polymer used in making plastics is usually a combination of additives, colorants, plasticizers, stabilizers, fillers, and reinforcements. These additives affect the chemical composition, properties, and mechanical properties of plastics and affect its cost.
7.2.2 Properties of Plastic
- Plastic is chemically unreactive:
Plastics do not react with air and water. Due to this, plastics are resistant to corrosion. They are also often unaffected by various chemicals. The plastic containers are used to store various kinds of materials, including many chemicals.
- Plastics are poor conductor of heat and electricity: They do not conduct heat or electricity, so they be used and insulators.
The handles of cooking utensils are made of plastic so that we can hold the hot cooking utensils safely.
- Plastics can be molded into different shapes:
Since plastics can be easily molded, they are used to make a large variety of articles having different shapes and sizes such as
Buckets, mugs, furniture, bags, sheets, bags, slippers, electrical fitting.
- Plastic and quite cheaper and easily made:
They are cheaper than metals. They are now widely used for making many of the household and industrial articles.
- Plastic is light, strong and durable
It is because of the lower price, easy availability, lightweight, good strength durability and corrosion resistance of plastic that the plastic container is preferred for storing food, water, milk, jams, pickles, squashes and soft drinks, being lighter than metals they are used in cars, aircrafts and spacecrafts.
7.2.3 Types of plastic Thermos softening and thermosetting plastics
Thermoplastic polymer: A thermoplastic is a resin that is solid at room temperature but becomes plastic and soft upon heating, flowing due to crystal melting or by virtue of crossing the glass transition temperature (Tg). Upon processing, usually via injection-moulding or blow-moulding-like processes, thermoplastics take the shape of the mould within which they are poured as melt, and cool to solidify into the desired shape. The significant aspect of thermoplastics is their reversibility, the ability to undergo reheating, melt again, and change shape. This allows for additional processing of the same material, even after being prepared as a solid. Processes such as extrusion, thermoforming, and injection moulding rely on such resin behaviour. Some common thermoplastic materials include polyethylene (PE), polycarbonate (PC), and polyvinyl chloride (PVC).
Thermosetting Polymer: A thermosetting resin, or thermosetting polymer, is generally a liquid material at room temperature which hardens irreversibly upon heating or chemical addition. When it is placed in a mould and heated, the most solidifies into the specified shape, but this solidification process includes the formation of certain bonds, called cross links, that hold the molecules in place and change the basic nature of the material, preventing it from melting. As a result, a there most, as opposed to a thermoplastic, cannot return to its initial phase, rendering the process irreversible. There most, upon heating, become set, fixed in a specific form. During overheating, there most tend to degrade without entering a fluid phase. Processes such as compression melding, resin transfer moulding, pultrusion, hand lay-up, and filament winding depend on thermosetting polymer behaviour. Some common there most include epoxy, polyimide, and phenolic, many of which are significant in composites.
7.2.4 Properties and applications of PVC and PET
Properties of PVC
- They are brittle and solid in nature and insoluble in Alcohol, but soluble in Hydrofluran,
- They show high amount of Hardness and show thermoplasticity, as the mechanical properties increase with the Increase in temperature.
- Heat Stability of PVC is quite poor as they begin to decompose once the temperature reaches 140 °C, the melting point of PVC is 160 °C.
- The elasticity of PVC reaches to 1500-3000 Mpa. They have ordinary friction.
- PVC shows good insulation properties, but its insulating property is less than that of Polyethylene.
- As the dielectric constant, dielectric loss tangent value and volume resistivity are high, the corona resistance is not very good, it is generally suitable for medium or low voltage and low frequency insulation materials
Applications of PVC
- PVC is a versatile material; hence they are used in window frames, drainage pipes, medical services, blood storage bags, wire insulation etc
- They are also applied to roofing materials, stationary automotive interiors, seat covers, resilient flooring.
- They are also into foot ware, credit cards, vinyl records cling films etc
- PVC has been used extensively in a wide range of construction products for over half a century. PVC's strong, lightweight, durable and versatile characteristics make it ideal for window profiles.
- PVC's inherent flame retardant and excellent electrical insulation properties make it ideal for cabling applications.
Properties of PET
- It has higher strength and stiffness than PBT
- It is very strong and lightweight & hence easy and efficient to transport
- They show good moisture barrier properties, and have good gas like (oxygen, carbon dioxide).
- They show good insulation, and have broad range of temperature from-60 to 130oC.
And also show good higher heat distortion temperature. It has low gas permeability, in particularly with carbon dioxide.
- PET is suitable for transparent applications, when quenching during processing
- They are practically shatter resistant thus helps in suitable glass replacement.
- They show recyclable properties, and are transparent to microwave radiation.
- PET is approved as safe for contact with foods and beverages by the FDA, Health Canada, EFSA & other health agencies.
- Excellent resistance to alcohols, aliphatic hydrocarbons, oils, greases and diluted acids
- Moderate resistance to diluted alkalis, aromatic & halogenated hydrocarbons
Polyethylene Terephthalate Applications
- Polyethylene Terephthalate is an excellent water and moisture barrier material, plastic bottles made from PET are widely used for mineral water and carbonated soft drinks
- Its high mechanical strength, makes Polyethylene Terephthalate films ideal for use in tape applications
- Non-oriented PET sheet can be thermoformed to make packaging trays and blisters
- Its chemical inertness, together with other physical properties, has made it particularly suitable for food packaging applications
- Other packaging applications include rigid cosmetic jars, microwavable containers, transparent films, etc.
7.2.5 Molding of plastic into articles: compression, extrusion and injection.
Steps involved in the Injection Molding:
The various stages of the injection molding process are carefully considered when analyzing part design, tool creation and efficient production of molded plastic products. There are a lot of factors and configurations which we won’t touch on here but the basic process is the same. Let’s start with the basics.
STEP 1: THE MOLD CLOSES
STEP 2: INJECTION
(The heated plastic is injected into the mold. As the melt enters the mold, the displaced air escapes through vents in the injection pins and along the parting line. Runner, gate and vent design are important to insure the mold is properly filled.)
STEP 3: COOLING
(Once the mold is filled the part is allowed to cool for the exact amount of time needed to harden the material. Cooling time is dependent on the type of resin used and the thickness of the part. Each mold is designed with internal cooling or heating lines where water is cycled through the mold to maintain a constant temperature)
STEP 4: PLASTICIZING THE RESIN
(While the part cools, the barrel screw retracts and draws new plastic resin into the barrel from the material hopper. The heater bands maintain the needed barrel temperature for the type of resin being used.)
STEP 5: EJECTION
(The mold opens and the ejector rod moves the ejector pins forward.
The part falls and is captured in a bin located below the mold.)
STEP 6: REMOVING THE RUNNER AND PACKAGING
Transfer Molding:
Transfer molding is similar to compression molding; however, the material is first placed in a transfer chamber prior to entering the mold. As in compression molding, thermosets that are cross-linked with heat are used for transfer-molding applications. Multiple cavities can be used within transfer molding since the material is entering the mold after the mold is closed. Since runners and sprues are present, shear is created. This facilitates heat needed for crosslinking and flow to the cavities. Transfer-molding machines are also generally positioned with the molds opening vertically.
Since the mold is closed and clamped prior to the material entering the mold, there is no presence of flash with transfer molded parts. The dimensions of the final parts are very accurate due to the flow of the polymer being gated. Another advantage is that the cure time is faster since there is the presence of shear flow, which creates heat. Inserts can also be used to create more complex parts than can be created by compression molding.
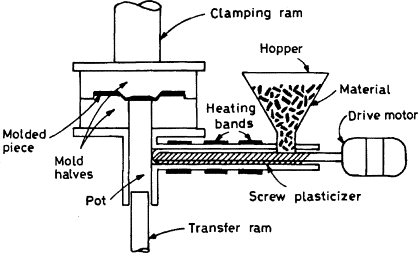
Advantages:
Shorter production cycles for higher weight parts
Transfer molding offers shorter production cycle times than traditional molding techniques, such as compression molding, as the compound preparation and product finishing time is greatly reduced. This means there is less cutting and flash. Cure times are reduced since the rubber enters the mould cavity at a higher and even temperature, and can thus begin to cure more quickly.
Tighter dimensional tolerance
The overall process also allows for much tighter tolerances, leading to more complex parts, which is difficult to achieve with compression molding. Given that the mould is not held open by surplus material spilling out of the cavity parting line, any excess holds the plunger open from the pot, therefore not affecting the actual part being produced.
Provides better uniformity
The pot and plunger design allow for more standardization and lower costs of tooling, and the process is more consistent than Compression molding, with less variables. The fact that the mould is closed before accepting the shot means that the parts are more dimensionally consistent across a production run.
Reduced tooling lead times compared to a full Injection tool
In relation to Injection Molding, Transfer Molding tooling offers much shorter lead-times with less ancillary features required. This means that the production run is ready faster with far less tooling costs.
More accurate and consistent than compression molding
Transfer molding allows for much sharper edges, since rubber enters the cavity at near the curing temperature. This vastly reduces any tendency for “backrinding”, which can be a risk with Compression molding. This occurs when rubber cures unevenly in a mould, and the action of expansion, cure and shrink tears off pieces of rubber around the split line of a mould. This is countered by increasing the width of the split line in a Compression tool, but greatly increases the overall deflashing necessary as a consequence. Transfer Molding generally eliminates this issue, and thus allows for sharper split lines.
Disadvantages:
More expensive tooling than a Compression mould
Due to the more complex nature of the design of the moulds, tooling investment can be somewhat higher.
Slower production cycle than an injection tool
The Transfer molding process is usually slower than an injection tool, sometimes limiting the overall production rate, as changeover times can be somewhat extended.
Manual handling of the piston can be a problem
The skill level is often proportionately higher and for larger parts or tools, manual handling can become somewhat of an issue.
Extrusion molding:
Extrusion molding (also known as plastification extruding) is a process that the stack of powders or the green body in die is pushed out to assume another form of green body or other final product under pressure. The cold extruding process is applied to mixtures of metal powders and organic binders, and extruding is performed at low temperatures (40–200 °C) to form the green body. The processes include material preparation, preprocessing, extruding, cutting, and reforming. The porous products can be obtained after drying, pre-sintering, and sintering of the extruded green body. It is an effective way to produce a long porous tube with a small diameter. The pretreatment of a mixture under pressure involves making full contact between the plasticizer and particle surfaces, to remove the gas inclusion and finally to ensure uniform density. Plasticizers have a large effect on a material’s properties. Therefore, certain requirements are needed for them, including that there should not be any reaction with porous materials during sintering, and that they should be removable, sticky, and have great pore-forming ability. The plasticizers in common use are olefin, amylum, and polythene alcohol. Powders will be subjected to pressure from the side wall, friction from either the powder and the wall or the extrusion shaft and the wall, in addition to the normal compression from the extrusion shaft. The key factors affecting the properties of green body extrusion are the types of powders, the particle shape and size, the plasticizer type and content, the precision of the mold, the pressure from extrusion, the extrusion speed, and the preheating temperatures. The selection of the preheating temperature depends on the optimal plastic used for the plasticizer at the selected temperature. The extrusion speed can be determined experimentally, and it is closely related to the particle size, shape, extrusion ratio, fluidity, extrusion force, and plasticizer. Higher extrusion speeds may cause the green body to crack.
Rubber, is a substance which is elastic in nature and is obtained from the sap of certain tropical plants, they are also derived from petroleum of natural gas (synthetic rubber). The unique properties of rubber are that they are Elastic, tough and shows resilience, rubber forms the basic constituent of tyres used in automobile vehicles, bicycles and aircraft. Rubber is found in the sap present on the bark of many tropical and sub-tropical shrubs and trees.
Among the most important synthetic Rubber are styrene-butadiene rubber, neoprene, butadiene rubber, polysulfide rubber and silicones. Synthetic rubbers, like natural rubbers, can be toughened by vulcanization and improved and modified for special purposes by reinforcement with other materials.
7.3.1 Classification
There are basically two broad categories into which the rubber types into which rubber can be classified. These are- Natural Rubber and Synthetic Rubber.
Natural Rubber
Natural Rubber is obtained naturally from the elastic material from the latex sap of trees, Natural rubber can also be vulcanized and processed into various other products of Rubber. The tropical and subtropical trees present in the regions of South East Asia, Amazon and Africa are known to produce the milky latex in the form of latex tubes. The rubber molecules present in these latex tubes consist of 5 carbon and 8 hydrogen atoms. Large number of rubber molecules are joined together to form a long chain like structure, this chain is called rubber molecules is called polymers, that helps rubber to be elastic in nature.
Synthetic Rubber
Rubber that is obtained from chemicals, that act as a substitute for the natural rubbers are called as synthetic polymers. Artificial elastomers form the synthetic rubber, Elastomers have the property of elasticity. There are numerous polymers that are used to make synthetic rubber types, the different synthetic polymers have different properties and are suited for specific need of rubber industries.
7.3.2 Isolation of natural rubber
Rubber is obtained primarily and naturally from the latex of Para rubber tree (Hevea brasilien-sis), that grows on plantations in many tropical countries. They are primarily obtained from the milky sap, or latex of the rubber bearing plants, they are also present in the cells and barks of the trees. which is grown on plantations in tropical countries. Malaysia is the largest producer of natural rubber (more than 40 percent of world production).
By tapping the bark of the tree, the latex is extracted. The natural rubber is isolated by the coagulation with formic acid, acetic acid or oxalic acid. The coagulum or the loose mass that is formed is washed with water and rolled on a mill forming sheets that are dried in specially smoked chambers, this helps the natural rubber to be resistant to oxidation and various microorganisms.
7.3.3 Vulcanization, properties and applications of Buna-S and Thiokol rubbers
Vulcanization, is a process natural rubber is treated with sulphur, the physical properties of both natural and synthetic polymers are improved. The rubber that has undergone vulcanization shows higher tensile strength and becomes resistant to abrasion or swelling,
Vulcanization is defined as a process of curing elastomers, in the process cross linking are formed between sections of polymer chains, thereby increasing their durability and rigidity. Changes are also brought about in the electrical and mechanical properties of the material. Vulcanization, in common with the curing of other thermosetting polymers, is generally irreversible.
The word vulcanization is derived from Vulcan, the Roman god of fire and forge.
Buna S
Buna s also known as Styrene-butadiene rubber (SBR), This is a copolymer that is produced from a combination of Styrene and butadiene, they are nothing but synthetic rubbers. And are known to be utilized more than any other synthetic rubber. They replace the natural rubber (polyisoprene) and are used in large scale in automobile and truck tires.
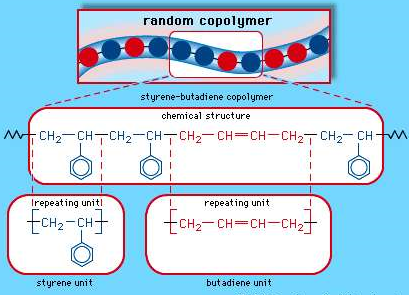
The random copolymer arrangement of styrene-butadiene copolymer.
Each coloured ball in the molecular structure diagram represents a styrene or butadiene repeating unit as shown in the chemical structure formula.
Applications: SBR is known to have good abrasion resistance, they are resistant to cracks and do not undergo ageing soon., They degrade by atmospheric Oxygen and ozone and is weakened by hydrocarbons. SBR replace the natural rubber due to their economic utilities.
T – THIOKOL RUBBER POLYSULFIDE
Polysulfides, also called polythioethers or Thiokols (T), are compounds with thioether functions in the backbone. They are typically liquid polymers that can be crosslinked by oxidizing the polymer’s terminals thiol groups (-SH) to disulfide (-S-S-) links. Common curing agents are oxygen donating compounds such as manganese dioxide, cumene hydroperoxide, and p-quinone dioxime. Other organic hydro peroxides, aldehydes and metallic paint driers can also function as curatives.
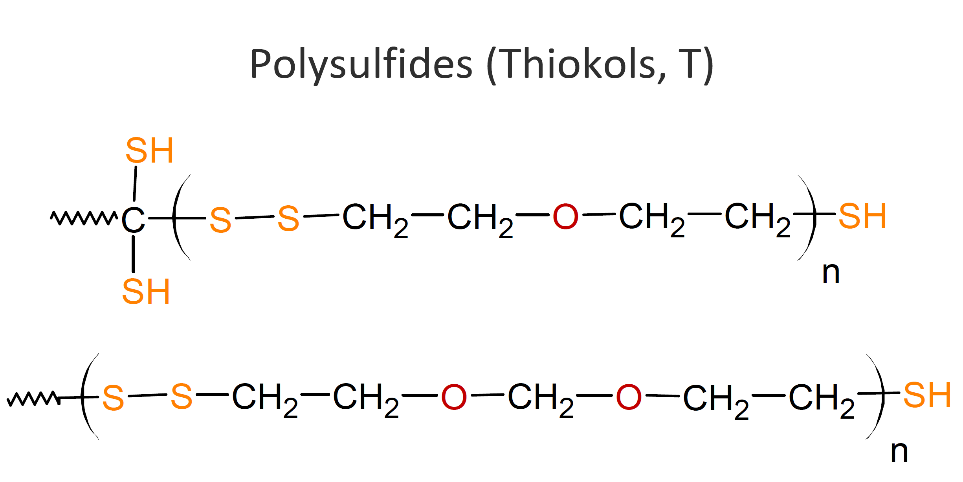
Thiokol elastomers show good resistance to oil, oxygen and ozone and other chemicals like aromatic hydrocarbons they exhibit gas permeability, and resistant to cracks. However, polythioethers have some major limitations. They do not stand out with respect to performance when compared to other elastomers, they show very poor resistance to heat, low tear. They are also not resistant to many polar solvents including (fatty) esters, mercaptans, amines, and chlorinated hydrocarbons.
APPLICATIONS
Polysulfides are widely used as the base polymer for sealants, caulks, and adhesives in various applications within the building & construction, aircraft, and automobile industry. Other industrial applications include highways sealants, molding / potting compounds, and concrete coatings.
The typical working temperature range is -45°C to +105°C. However, some grades can withstand temperatures up to 150/170°C (for a short time).
7.3.4 Biodegradable Polymers: Introduction, examples with applications.
(polyhydroxy butyrate – hydroxyl Valente)
Biodegradation: -
It is a process of converting polymer material into harmless simple gaseous products by the action on enzymes of microorganism and water.
A polymer which can be converted to harmless gaseous products by action of biological enzyme and water, is called as biodegradable polymers 3 components are important in the biodegradation of organic polymers.
A) Organisms: - like pseudomonas, bacillus, protozoa, acetobacter, various fungi, etc.
B) Environment: - suitable temp, moist condition, presence of salts, suitable ph.
C) Nature of polymer: -
- Polymer chain should contain bond which can be hydrolysed or oxidized by the enzyme action o, s.
- High aromatic they are tough for degradation
Hydrophilic chain backbone of polymer contains O, N,3. Atoms easily degrade.
3. Hydrophilic end groups
4. More amorphous nature of polymer
5. Small size of polymer material
Example: - PHBV
It is a co – polymer of hydroxy butyric acid and 3 hydroxy valeric acid. It is produced by fermentation of glucose by acaligeneseutrophus species.
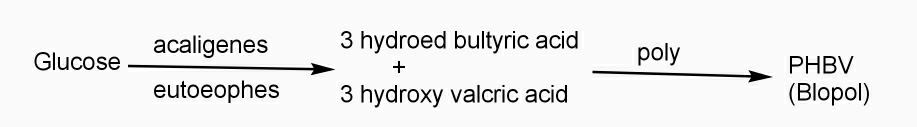
STRUTURE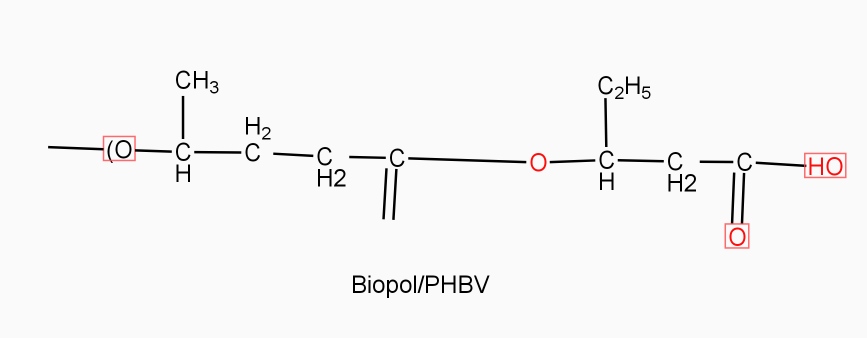
Properties: -
- It is highly crystalline, soluble in chloroform
- It has melting point 180° c
- It is thermos softening polymer ,soft, flexible
Limitations: -
- Such polymers are very costly
- They cannot manufacture on a large scale
- Don’t possess high mechanical strength
Applications: -
- Used for moulded articles
- Used for films for packing and lamination
- It is used in medical field – for organs transplants, surgical, and orthopaedic, operations.
- It is used in agriculture field – for manufacturing fertilizers and pesticides.
Reference:
1. Engineering Chemistry, Jain and Jain, Dhanpat Rai & Co
2. Engineering Chemistry, M. Subha Ramesh, Dr. S. Vairan-Ed.-IInd Wiley
3. Instrumental Methods of chemical analysis, Chatwal and Anand,Himalaya Pub House
4. Industrial Chemistry, B.K.Sharma,Goyal
5. Chemistry for Engineers, Rajesh Agnihotri, Wiley
6. Fundamentals of Engineering Chemistry, S.K.Singh, New Age Int.
7. Engineering Chemistry (NPTEL Web book), B. L. Tembe, Kamaluddin & M. S. Krishnan.
8. A text book of Engineering Chemistry, S.S. Dara, S S Umare, S Chand
9. A text book of Engineering Chemistry, Shashi Chawala, Dhanpat Rai & Co
10. A text book of Experiments and Calculations in Engineering Chemistry, S.S. Dara. S Chand