Unit- 8
Modern Analytical Techniques
Molarity: Molarity of a given solution is defined as the total number of moles of solute per litre of solution. The changes in physical properties of the system like pressure and temperature affect the molarity of the solution, the change in the physical properties causes a change in the volume of the system. Molarity is represented by M, which is termed as molar. One molar is the molarity of a solution where one gram of solute dissolved in a litre of solution. The total volume of the solution is taken as the solute and the solvent blend together to form the solution.
Molarity Formula:
The equation for the calculation of molarity is the ratio of the moles of solute whose molarity is to be calculated and the volume of solvent used to dissolve the given solute.
M=nV
Here,
M is the molality of the solution that is to be calculated
n is the number of moles of the solute
V is the volume of solution given in terms of litres
Normality:
Normality is the expression used to measure the concentration of the solution. They also refer to the equivalent concentration of the solution and is abbreviated as ‘N’. Normality has its importance in an acid base titration, or in titrations and also in the measure of the reactive species in a solution.
As per the standard definition, normality is described as the number of gram or mole equivalents of solute present in one litre of a solution.
Normality Formula
- Normality = Number of gram equivalents × [volume of solution in litres]-1
- Number of gram equivalents = weight of solute × [Equivalent weight of solute]-1
- N = Weight of Solute (gram) × [Equivalent weight × Volume (L)]
- N = Molarity × Molar mass × [Equivalent mass]-1
- N = Molarity × Basicity = Molarity × Acidity
Mole fraction:
Mole fraction represents the number of molecules of a particular component in a mixture divided by the total number of moles in the given mixture. In other words, it’s a way of expressing the concentration of a solution.
Mole Fraction formula
The molar fraction can be represented by X. If the solution consists of components A and B, then the mole fraction is,
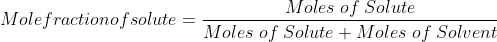
= 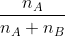
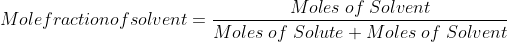
= 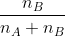
Therefore, the sum of mole fraction of all the components is always equal to one.
8.2.1 Introduction of Chromatography
Chromatography is the technique which is used for the separation, purification and testing of the compounds. Chromatography word is derived from the Greek word ‘Chroma’ that means color. Chromatography helps in separating the mixture in its components between in two phases. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down to move slower than the mobile phase. The movement of the components in the mobile phase is controlled by the significance of their interactions with the mobile and stationary phases. Because of the differences in factors such as the solubility of certain components in the mobile phase and the strength of their affinities for the stationary phase, some components will move faster than others, thus facilitating the separation of the components within that mixture.
Types of Chromatography
Chromatography is divided into 4 different parts:
1) Thin Layer Chromatography
2) Adsorption Chromatography
3) Partition Chromatography
4) Column Chromatography
Thin Layer Chromatography: The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbent like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
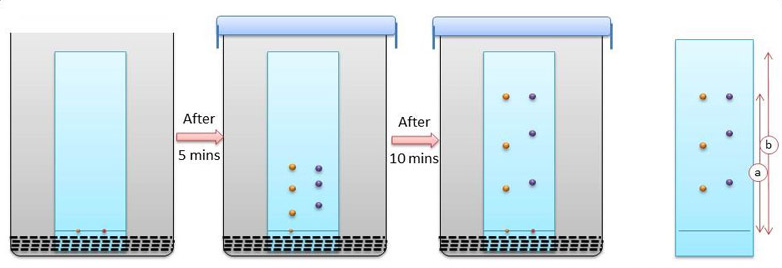
Adsorption Chromatography: This chromatography is one of the oldest types of chromatography. It utilizes a mobile liquid or gaseous phase that is adsorbed onto the surface of a stationary solid phase. The equilibrium between the mobile and stationary phase accounts for the separation of different solutes.
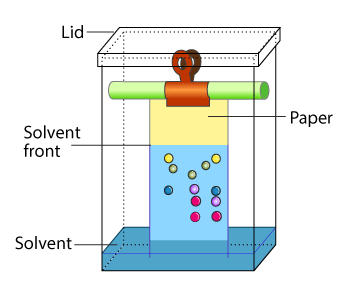
Partition Chromatography: Partition chromatography is based on the continuous differential partition of components of a mixture between stationary and mobile phases. Paper chromatography is a type of partition chromatography. In the paper chromatography a special quality paper is used that is called as the chromatography paper. Chromatography paper contains water trapped in it, which acts as the stationary phase.
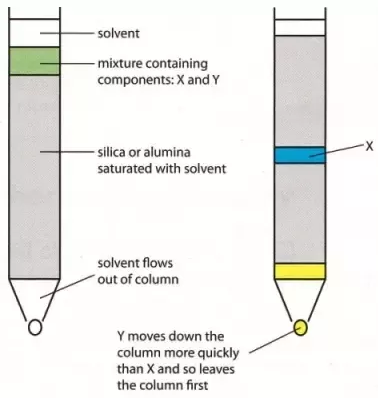
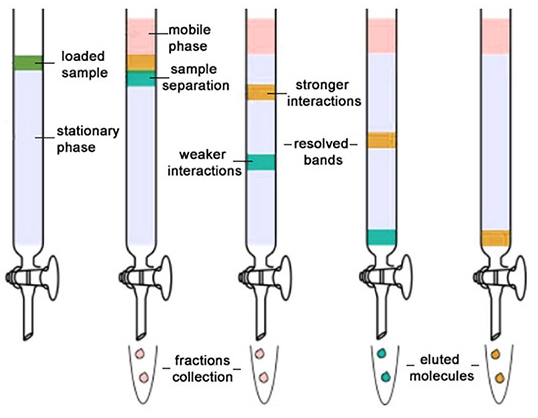
Column Chromatography: Column chromatography is the technique used to separate the components of a mixture using a column of suitable adsorbent packed in a glass tube. The mixture is placed on the top of the column, and an appropriate eluant is made to flow down the column slowly.
Depending upon the degree of adsorption of the components on the wall adsorbent column, the separation of the components takes place. The component with the highest absorptive is retained at the top, while the other flow down to different heights accordingly.
8.2.2 Gas-liquid Chromatography (GLC):
This is the one of the fastest separation method, which allows for the quantitative determination of each compound of the mixture. In the Gas-Liquid chromatography the process begins by the bringing of sample by the syringe to the heated injection chamber. The sample is passing through the nitrogen gas in the column. The same column is has the thin film of liquid. After the separation of the carried gas the separated mixture flows in detector which activates a recorder. Traces a series of peaks on chromatogram.
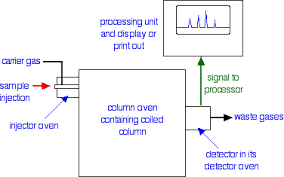
Basic Principle:
Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase is separating from each other while moving with the aid of a mobile phase. The factors effective on this separation process include molecular characteristics related to adsorption (liquid-solid), partition (liquid-solid), and affinity or differences among their molecular weights because of these differences, some components of the mixture stay longer in the stationary phase, and they move slowly in the chromatography system, while others pass rapidly into the mobile phase and leave the system faster.
Instrumentation and Applications
1) The chromatographic technique is used for the separation of amino acids, proteins and carbohydrates.
2) It is helpful for the qualitative and quantitative analysis of complex mixtures.
3) It is also used for the analysis of drugs, hormones, vitamins and brain amines.
4) This technique is also helpful for the determination of molecular weight of proteins.
5) It is helpful in detection of phenolic compounds in drinking water.
6) It is helpful in detection of endogenous Neuro peptides in extracellular fluid of brain etc.
8.2.3 Thermal analysis
Definition of TGA
Thermogravimetric analysis (TGA) is a thermal analysis technique that measures the weight change in a material as a function of temperature and time, in a controlled environment. This is very useful to investigate the thermal stability of a material, or to investigate its behaviour in different atmospheres (e.g., inert or oxidizing). It is suitable for use with all types of solid materials, including organic or inorganic materials.
Instrumentation and application of TGA
Thermogravimetric analysis (TGA), is mainly conducted on an Instrument called thermogravimetric analyzer, this instrument measures the mass continuously while the temperature of the sample is changed with time. In the thermogravimetric analysis mass, temperature and time are considered base measurements.
The instrument consists of a precision balance that holds a sample pan, placed inside a furnace where the temperature can be controlled. For a thermal reaction to occur the temperature is increase at a constant rate, the thermal reaction may occur at various atmospheres like vacuum, ambient air, corrosive gases, self-generated atmosphere or liquids etc., also in a range of pressure like high vacuum, high pressure, constant or controlled pressure.
The thermogravimetric data that is obtained from a thermal reaction is compiled and plotted, on the y axis is the percentage initial mass or the mass verses the temperature and time on the X axis. The plot obtained is often smoothed and is called the TGA curve. The first derivative of this curve is useful in interpreting the differential thermal analysis.

A Typical TGA system
Advantages:
They can be used, for materials that are characterised through decomposition patterns especially in polymers.
TGA is used to evaluate thermal stability of a material
They also provide upper use temperature of a material
Reference:
1. Engineering Chemistry, Jain and Jain, Dhanpat Rai & Co
2. Engineering Chemistry, M. Subha Ramesh, Dr. S. Vairan-Ed.-IInd Wiley
3. Instrumental Methods of chemical analysis, Chatwal and Anand,Himalaya Pub House
4. Industrial Chemistry, B.K.Sharma,Goyal
5. Chemistry for Engineers, Rajesh Agnihotri, Wiley
6. Fundamentals of Engineering Chemistry, S.K.Singh, New Age Int.
7. Engineering Chemistry (NPTEL Web book), B. L. Tembe, Kamaluddin & M. S. Krishnan.
8. A text book of Engineering Chemistry, S.S. Dara, S S Umare, S Chand
9. A text book of Engineering Chemistry, Shashi Chawala, Dhanpat Rai & Co
10. A text book of Experiments and Calculations in Engineering Chemistry, S.S. Dara. S Chand