Unit 7
Effective Nuclear Charge
- Effective Nuclear charge
The term effective Nuclear charge is the net total charge that an electron experiences in an atom in the presence multiple electrons. The effective nuclear charge may be estimated by the equation:
Zeff = Z - S
Where Z is the atomic number and S is the number of shielding electrons.
Electrons of higher can possess different electrons of lower energy between the electron and the nucleus, thereby lowering the positive charge experienced by the high energy electron.
The shielding effect is named as it gives a balance between the repulsion between valence and inner electrons the attraction of valence electrons. The shielding effect also explains the trend in atomic size followed in the periodic table and also explains why valence electrons are readily removed from an atom.
Periodic table
- Variation of s, p, d and f orbitals energies of atoms in the Periodic Table
Orbitals are regions of space where electrons are most likely to be found in an atom. These orbitals are designed as s, p, d, f among others. The energy increases as you move up the orbital in such a way that S orbital has the lowest energy and f orbital has highest energy as the energy levels increases the electrons are located further away from the nucleus.
- S-orbitals
An S orbital is spherical symmetrical around the nucleus of an atom like a hollow ball with nucleus at the centre. As the levels of energy increase, the electrons are located further away from the nucleus, so the orbitals get bigger the order of size is 1s<2s<3s< ------ as shown 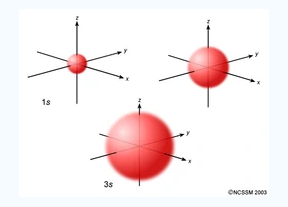
Fig.2: The figure shows that as the energy levels increase, the electrons move further away from the nucleus, as a result the orbitals get bigger in size the order of size is 1s<2s<3s<…
- p orbitals
Unlike s orbitals, a p orbital points in a particular direction, at any one energy level we have three absolutely equivalent p orbitals pointing mutually at right angles to each other they are given symbols px , py, pz. . The p-orbitals at the second energy level are called 2px, 2py,2pz and so on. All levels except the first energy level have p orbitals.
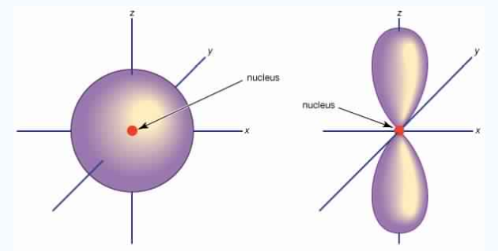
Fig.3: At any one energy level, we have absolutely three equivalent p orbital pointing
Mutually at right angles to each other, They are arbitrarily given the symbols px, py, pz .
At any one energy, we have three absolutely equivalent p orbitals pointing mutually at right angles to each other. These are arbitrarily given the symbols px, py, pz, which is for convenience. The p orbitals at the second energy level are called 2px,2py,2pz. There are similar orbitals at subsequent levels: 3px,3py,3pz….and 4px, p4py,4pz and so on. All levels except the first have p orbitals.
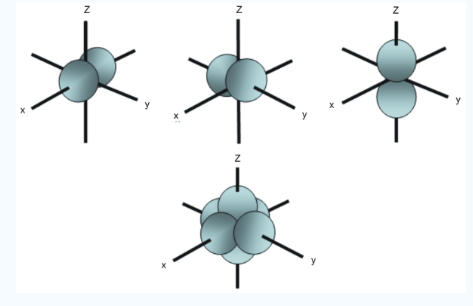
Fig.4: In addition to s and p orbitals, there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels ,at the third level, there are a aet of five d orbitals as well as 3s and 3p orbitals, at the third level there are a total of nine orbitals.
- d orbital
In addition to s and p orbitals, there are 2 other sets of orbitals which are available for electrons to inhabit at higher energy levels as well as the 3s and 3p orbitals at the third level there are now a total of nine orbitals altogether.
3dxy, 3dxz, 3dyz, 3dx2-y2, 3dz2
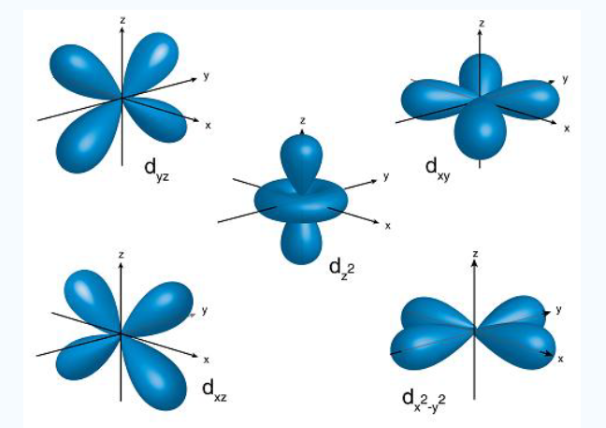
Fig. 5: The first group contains 3dxy,3dxz and3dyz orbitals, the names specify that these orbitals Lie in the x-y plane, the x-z plane and the y-z plane. Each lobe has four lobes and each lobe points between .Two axes and not along them. The second group contains the 3dx2-y2and3dz2 orbitals, these lobes point along various axes the 3dx2-y2 looks exactly like the first group, except that the lobes are pointing along .The x and y axes, not between them. The3dz2 looks like a p orbital...:
- f orbital
At the fourth and higher levels there are 7f orbitals in addition to the 4s, 4p and 4d orbitals. Counting the 4s, 4p and 4d orbitals. This makes a total of 16 orbitals in the 4th level.
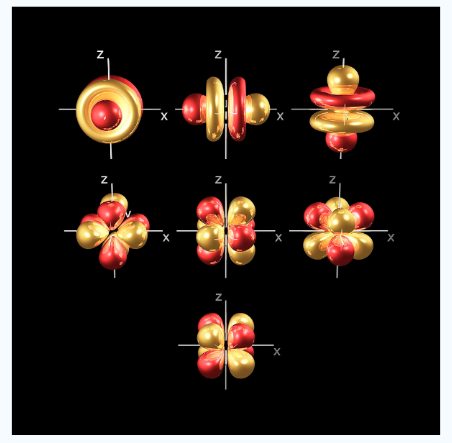
Fig.6: counting the 4s,4p,4d orbitals, this makes a total of 16 orbitals in the fourth level.the
s,p,d and f orbitals are more complicated at higher energy levels.
- Electron Configuration
Electron configuration holds the key to the chemical world. Our generation understands the chemical properties of organic and inorganic compounds based on the electronic configuration of the constituent elements.
Therefore, in a reaction the chemical bonding is the arrangement of electrons of reactant atoms. Classification of periodic table is based on the electron configuration of the elements.
The 3S orbital has lower energy than 3P orbitals which again has a lower energy than 3d orbitals. But the orbitals belonging to a particular type (P or d or f) will have an energy equal of an atom or an ion.
Rules for writing electronic configuration
The electron configuration of elements or its ions should be according to the following rules
- The maximum number of electrons in the main quantum shell = 2n2. Where n = principal quantum.
- Again we see that the principal quantum shell divided into sub shell s, p, d, f and the maximum capacity of electrons in a subshell = 2(2l+1).
Where l = 0, 1, 2, 3 for s, p, d, f orbitals respectively. - The orbitals are filled up in the order of increasing energy of an electron. Thus orbitals with the lowest energy are filled up first while the orbitals with highest energy are filled up in the end.
- Electrons will turn to maintain maximum spin. Thus electrons with similar spin occupied first will prefer to remain unpaired.
- According to Hund’s rule, electrons are filling in the orbital with maximum spin multiplicity.
- Spin pairing occurs only when empty orbitals of same energy are not available for work.
Electron configuration and energy levels
An easy way to remember these energy levels is provided below this diagram
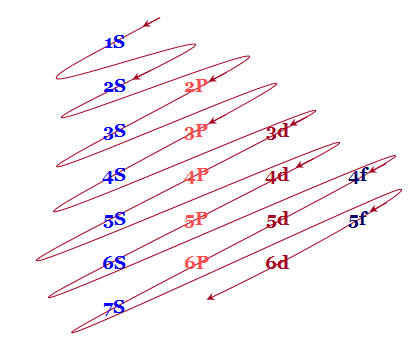
Fig.7: The figure shows an increase in energy every orbital. S orbital have the lowest energy, energy continuously increases in each block as we move, downward in group of left to right across the period.
Electron shells
- The different orbitals originating from the same principal quantum number n are written in the horizontal lines.
- The parallel inclined lines are drawn through the orbitals according to the above picture. Therefore filling up the different orbitals by electrons will follow these lines.
According to this diagram, the energy levels are
1S < 2S < 2P < 3S < 3P < 4S < 3d < 4P < 5S < 4d < 5P < 6S < 4f < 5d < 6P < 7S < 5f…
- Atomic size
Atomic radii or size can be defined in the most simplified manner as the radii (or half the "width") of the Spherical atoms. Nonbonding atoms have a much larger, more indefinite or "fuzzy" radius, therefore When atomic radius is described as a periodic trend, the thing that comes to mind is bonding atomic radius. These are the radii of atoms that are chemically bonded to each another. So, if the bond between two Cl atoms in Cl2 is 1.99 angstroms, resulting in chlorine's bonding atomic radius as about 0.99 angstrom These values are then used to estimate bond lengths between different elements in molecules. |
Atomic radius generally increases from top to bottom down a group. This generally occurs due to the Increase in the principal quantum number, (n). Therefore when we go further down a column, the outer Electrons move much further away from the atom, making the atom thus larger. Generally the Atomic radius decreases as we move from left to right across a period. This occurs mainly Due to effective nuclear charge. The greater the effective nuclear charge, the more will be the Attraction felt by the outer electrons from the nucleus, and the closer the outer electrons Are to the nucleus, making the atom smaller.
|
Ionic radii are the radii of ions of elements. These distances are based on distances that exist between Ions in ionic compounds. Cations, or positively charged ions, are smaller in size than their "parent" atoms. This is because Cations are formed when the outermost orbitals become empty due to absence of electrons. This also Decreases electron-electron repulsions. Therefore, the resulting ions are smaller as there are not as many Orbitals that are occupied and the effective nuclear charge affecting the remaining electrons increases, Pulling electrons in more closely. Anions, or negatively charged ions, are larger in size than their "parent" atoms. This is because Of the addition of electrons form these ions, increasing electron-electron repulsions, making the Electrons spread out more effectively. Also, effective nuclear charge felt by the outermost Electrons decreases. For ions that carry the same charge (i.e. from parent elements of the Same group), size increases from top to bottom as we go down a group.
|
Oxidation states, Hybridization and molecular geometries
- Electron affinity and Electronegativity
- Electron affinity
Electron affinity reflects the ability of an atom to accept an electron. It is the energy change
Which occurs when an electron is added to a gaseous atom. Atoms with strong effective nuclear
Charge have greater electron affinity.
The reaction that occurs when an atom takes an electron may be represented as:
X + e− → X− + energy
Another way to define electron affinity is as the amount of energy needed to remove
An electron from a singly charged negative ion:
X− → X + e−
Key Takeaways: Electron Affinity Definition and Trend
- Electron affinity is the amount of energy required to detach one electron from a
Negatively charged ion of an atom or molecule.
- It is indicated using the symbol Ea and is usually expressed in units of kJ/mol.
- Electron affinity follows a particular path on the periodic table. It increases when it moves down a column or group and also shows an increase when it moves from left to right across a period or row (except for the noble gases).
- The value may be either positive or negative. A negative electron affinity means energy must be added in order to attach an electron to the ion. Here, the capture of electrons is an endothermic process. If electron affinity is positive, the process is exothermic and occurs spontaneously.
- Electronegativity
Electronegativity is the property of an atom which increases with its tendency to attract the electrons of a bond. When two bonded atoms that have similar electronegativity values as each other, they share electrons equally in a covalent bond. Usually, the electrons present in a chemical bond are more attracted to one atom (the more electronegative one) than to the other one. This results in a polar covalent bond. If the electronegativity values are very different, the electrons do not show any sharing. One atom essentially takes the bond electrons from the other atom, forming an ionic bond.
Electronegativity is a property of an atom present within a molecule, rather than an fundamental property of an atom by itself. Thus, electronegativity varies depending on an atom's environment or surroundings.
However, most of the time an atom displays similar behaviour in different situations. Factors that affect electronegativity include the nuclear charge and the position and number of electrons in an atom.