UNIT4
Green Chemistry and Battery Technology
Introduction:
The term used for green chemistry is sustainable chemistry. The area of chemistry which deals with the design of products that reduces the generation of hazardous products. Green chemistry deals with improving the environment, it reduces the chemical action on the environment it includes the consumption of non-renewable resources. “Green Chemistry is the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products.”
Principles of Green Chemistry:
There are following 12 major principles of green chemistry:
(i) Preventing Waste
(ii) Maximize atom economy
(iii) Less hazardous chemical syntheses
(iv) Designing safer chemicals
(v) Safer solvents and Auxiliaries
(vi) Increases Energy efficiency
(vii) Uses renewable feedstock
(viii) Reduce derivatives
(ix) Use catalysts, not stoichiometric reagent
(x) Design chemicals and products to be degraded after use
(xi) Real time analysis to prevent pollution
(xii) Minimize the potential for accidents
Significance:
(i) Releases less hazardous chemical to air that result in the lesser damage to human health.
(ii) Ensures the safety for workers in the chemical industry.
(iii) Due to its hazardous free nature it causes lesser hazardous impact on the environment that intends result the lesser harm to the plants and animals.
(iv) This allows the replacement of purchased feedstock by waste product.
(v) The plants and animals suffer lesser harm from toxic chemical in teh environment.
Supercritical Fluids: This technology is involved in the wide variety of industrial applications. There are many sectors like food, cosmetics, pharmaceutics, waste treatment which are involved in this. The related superficial fluid processes include extraction, impregnation, formulation and waste treatment among others. Super critical CO2 is a fluid state of CO2 where it is held or above the critical temperature and pressure.CO2 shows the dual property in the nature i.e., it behave like gas and dry ice as a solid. At the standard temperature and pressure CO2 shows the property of gas while when it is cooled or the sufficient amount of pressure is exerted on it then CO2 behaves like as a solid dry ice while on increasing the temperature and pressure from its standard temperature and pressure and reached upto the critical point or above that then it adopt the midway property between gas and a liquid or it behave as a superficial fluid above its critical temperature. CO2 is majorly used as chemical extraction that is why it becomes an important to be used in commercial and industrial solvent.
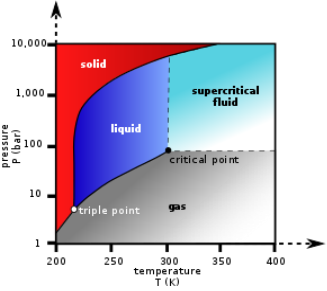
The temperature and pressure phase diagram of carbon di oxide.
Industrial Application:
CO2 is highly used by coffee manufacturers; they are looking forward from classic decaffeinating solvents, because of real or perceived danger related to their use in food preparation. CO2 are forced through the green coffee beans which are then sprayed with water at high pressure to remove caffeine’s further the same can be resold by distillation.
This CO2 can be used as more environment friendly solvent for dry cleaning over traditional solvents. This CO2 is used as the extraction solvent for the creation of essential oils and other herbal distillates.
Biocatalysts: The metabolic transformation of chemicals to produce new chemicals for industrial purposes is called as Biocatalysis. Biocatalyst refers to the living system or their parts to speed up chemical reaction. Modern biotechnology has made the production of modified or non-natural enzymes possible. “Biocatalysis is defined as the use of natural substances that include enzymes from biological sources or whole cells to speed up chemical reactions.”
Concepts of carbon credits: Carbon credits came in existence due to the need of controlling the emissions. The regular and abandon release of oxygen in the atmosphere leads to the formation of this. This was the first attempt made to reduce the emission of greenhouse and harmful gases coming from industrial activity. Carbon Credit is nothing but the tradable certificate that gives the permit for the emission of carbon dioxide or other green houses to the holder.
A carbon credit is a generic term for any tradable certificate or permit representing the right to emit one tonne of carbon dioxide or the equivalent amount of a different greenhouse gas. Carbon credits and carbon markets are a component of national and international attempts to mitigate the growth in concentrations of greenhouse gases (GHGs).
There are 2 major markets for carbon credits i.e., compliance market credit and Verified market credits.
Kyoto’s Flexible mechanism:
The Kyoto protocol provides the three major mechanisms that enable countries or companies tn developed countries to acquire greenhouse gas reduction credits.
(i) Under the proper agreement the developed country with relatively high costs of domestic greenhouse reduction would set a project in another developed country.
(ii) Under the Clean Development Mechanism, a developed country can sponsor a greenhouse gas reduction project in developing countries.
(iii) Under the Emission Trading countries can trade in international carbon credit market to cover their shortfall in Assigned amount units.
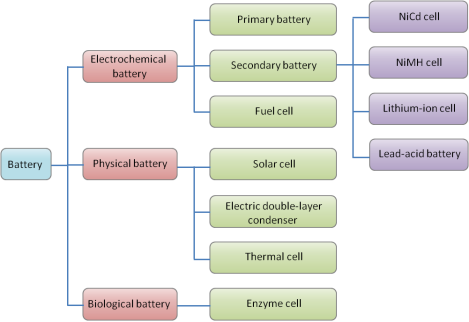
Battery is the device which helps in the conversion of chemical energy into electrical energy. A battery is an arrangement of cells whether that may be two or beyond than that, usually they are connected in series or parallel for the successful supply of current.
Primary cells:
Primary cells are those cells in which the electrical energy can be obtained from the chemical energy as long as it consists the active materials in it. After the complete consumption of active material from it the cell cannot be used any more or they must be discarded.
Secondary cells:
Secondary batteries are rechargeable batteries which can be recharged after its use. The redox reaction gets reversed during the recharge process. Since the electrical energy brings about the chemical change, it is converted into chemical energy. Thus, electrical energy is stored in the form of chemical energy and utilized for supplying the current when needed. Secondary cells are also known as storage cells.
Reverse Batteries:
In Reverse batteries, a key component is separated from the rest of the battery prior to activation. Usually the electrolyte is the component that is isolated. Batteries which use highly active component material are designed in this form to withstand deterioration in storage and to eliminate self-discharge prior to use. The reserve design is also used for batteries required to meet extremely long or environmentally severe storage requirements.
Energy Density:
Energy Density is defined as the amount of energy stored in the given system of space per unit volume. The higher the energy density of a system the greater the amount of energy stored in the mass. There are several different types of energy content. One is the theoretical total amount of thermodynamic work that can be derived from a system, with a given temperature and pressure for the surroundings. This is called energy. Another is the theoretical amount of work that can be derived from reactants that are initially at room temperature and atmospheric pressure. This is given by the change in standard Gibbs free energy. But as a source of heat or for use in a heat engine, the relevant quantity is the change in standard enthalpy or the heat of combustion.
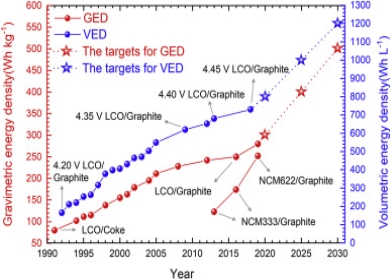
Batteries with high theoretical energy densities.
There are two kinds of heat of combustion:
(i) The higher value (HHV), or gross heat of combustion, includes all the heat released as the products cool to room temperature and whatever water vapor is present condenses.
(ii) The lower value (LHV), or net heat of combustion, does not include the heat which could be released by condensing water vapor, and may not include the heat released on cooling all the way down to room temperature.
Power Density:
The measure of output per unit volume. Power density usage is not as common as the energy density use. The condition at which the system has a high-power density then it can output large amount of energy based on its volume.
E.g.- a tiny capacitor may have the same power output as a large battery. Because the capacitor is so much smaller, it has a higher power density. Since they release their energy quickly, high power density systems can also recharge quickly.
Secondary Battery: Secondary batteries are rechargeable batteries which can be recharged after its use. The redox reaction get reversed during the recharge process. Since the electrical energy brings about the chemical change, it is converted into chemical energy. Thus electrical energy is stored in the form of chemical energy and utilized for supplying the current when needed. Secondary cells are also known as storage cells.
Nickel-Cadmium: Nickel cadmium battery is a type of rechargeable battery which uses nickel oxide hydroxide and metallic cadmium as electrodes. The maximum recharge rate for Ni-Cd battery varies by size.
Voltage: Zinc carbon primary cells and Nickel cadmium cells are not preferably exchangeable as because the potential of Zinc Carbon primary cells are greater than that of Ni-Cd cells. As many electronic devices are designed to work with primary cell that may discharge as low as 0.90 to 1.0V per cell.
Charging: Depending on the cell manufacturing Ni-Cd batteries can be charged in different ways. The charge rate is measured based on the percentage of the amp-hour capacity the battery is fed as the steady current over the duration of charge.
On fully charging the Ni-Cd batteries the batteries consist a nickel oxide hydroxide positive electrode plate, a cadmium negative electrode plate, a separator and an alkaline electrolyte. Nickel Cadmium consist of nickel wire gauze and electrode grids. The anode grid consists of a mixture of spongy cadmium with 78% cadmium hydroxide, 18% iron, 1% nickel and 1% graphite. The cathode grid contains nickel hydroxide (80%), cobalt hydroxide (2%), graphite (18%) and traces of barium compound. Graphite increases the conductivity, the cobalt and barium compounds increases the efficiency of active material and also the cycle life. 6M KOH is the electrolyte. 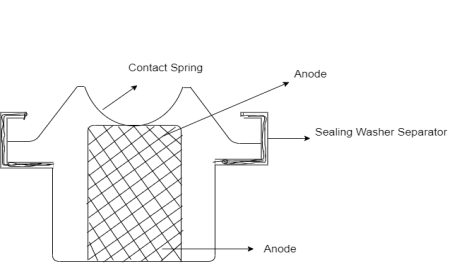
Electrode Reaction:
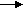 On anode, Cd + 2OH- Cd(OH)2 + 2e-
On anode, Cd + 2OH- Cd(OH)2 + 2e-
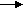 On cathode end, 2Ni(OH)3 + 2e- 2Ni(OH)2 + 2OH-
On cathode end, 2Ni(OH)3 + 2e- 2Ni(OH)2 + 2OH-
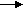 Cd + 2Ni(OH)3 2Ni(OH)2 + Cd(OH)2
Cd + 2Ni(OH)3 2Ni(OH)2 + Cd(OH)2
Applications: These are used in pocket calculators, photo flash units, cordless garden tools, alarm, emergency lighting, instrument etc.
Lithium-ions battery: Lithium metals are used as a battery anode material because of its light weight, low electrode potential, high electrochemical equivalence and good conductivity. These batteries are type of rechargeable battery. These types of battery are commonly used for portable electronics and electric vehicles. Lithium batteries are classified into primary and secondary batteries. Primary battery is not chargeable and the secondary battery is chargeable. On the basis of cathode material used lithium primary cells are classified as:
(i) Soluble-cathode cells: In this type of cells liquid or gaseous cathode material, such as sulphur dioxide or thionyl chloride.
(ii) Solid-cathode cells: These materials used solid material for cathode substance such as V2O5, MnO2, CuO etc.
(iii) Solid electrolyte cell: This type of cells use electrolytes in the solid form itself as the cathode. E.g.- PbI2, PbS etc. Are used as solid electrolyte cathodes.
Advantages of Lithium batteries:
• High cell voltage, up to above 4 V, depending on the cathode material. This is because of the very negative electrode potential of Li/ Li+.
• High energy density due to the low atomic mass of lithium. 1F is released by the dissolution of 7 g of the metal.
• Operation over a wide temperature range, from about 70 to – 40°C.
• Flat discharge characteristics-constant voltage and resistance through most of the discharges of many lithium cells.
Fuel Cells: A fuel cell is an electrochemical that converts chemical energy of a fuel and an oxidizing agent into electricity through a pair of redox reaction. Fuel cells can produce electricity continuously as long as fuel and oxygen are supplied. Alkaline fuel cells are also called as the Bacon fuel cell. This is one of the most developed fuel cell technologies. This cell consumes hydrogen and pure oxygen to produce potable water, heat and electricity. This cell produces power through a redox reaction between hydrogen and oxygen.
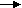 H2 + 2OH- 2H2O + 2e-
H2 + 2OH- 2H2O + 2e-
Producing water and releasing electrons. The electrons flow through an external circuit and return to the cathode, reducing oxygen in the reaction:
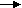
 O2 + 2H2O + 4e- 4OH-
O2 + 2H2O + 4e- 4OH-
Producing hydroxide ions. The net reaction consumes one oxygen molecule and two hydrogen molecules in the production of two water molecules. Electricity and heat are formed as by-products of this reaction.
Principle:
- Alkaline fuel cell works optimally at 80oC using relatively inexpensive material.
- A single AFC consist of 2 electrodes with liquid KOH electrolyte between them.
- The hydrogen fuel is applied to the anode electrode, while oxygen from the air is supplied to the cathode.
- Voltage between the anode and the cathode of a single fuel cell is between 0.9V and 0.5V depending on the load.
![Schematic representation of an alkaline fuel cell (AFC) [2]. | Download Scientific Diagram](https://glossaread-contain.s3.ap-south-1.amazonaws.com/epub/1643158540_1423798.png)
Application:
(i) Alkali cell provides drinking water for astronauts
(ii) NASA alkali fuel cell for the space shuttle fleet as well as Apollo program.
(iii) It is use as a power source in experimental purpose.
Advantage:
(i) Activation over voltages at the cathode is usually less than acid electrolyte fuel cell.
(ii) Electrode are made of normal materials, there is no use of precious metal or costly material in it.
(iii) Good Power density
(iv) Low temperature operation
(v) Mobile electrolyte fuel cell can easily cooled by circulated hydrogen.
Limitations:
(i) They need to installed in the CO2 free environment.
(ii) Preparation of the electrodes with noble metal catalyst becomes very expensive.
(iii) Diaphragm made of asbestos but this material is hazardous for health.
Battery Technology:
Battery is the device which helps in the conversion of chemical energy into electrical energy. A battery is an arrangement of cells whether that may be two or beyond than that, usually they are connected in series or parallel for the successful supply of current. The spontaneous redox reaction which forms the basis of voltaic cell is conveniently used in making commercial cells. A useful commercial cell must fulfil the following specifications:
(i) Portability
(ii) Should be light in weight and compact
(iii) It should be pocket wise price with good electric supplies
(iv) Low cell resistance
(v) High sustained power output
(vi) It should store high energy content per unit mass and per unit volume
(vii) Wide temperature range of operation
(viii) Low initial cost
(ix) It must be sealed and leak proof
(x) Rugged and resistant to abuse
(xi) It should be safe in rough use
Types of battery: