Unit - 4
Introduction to Advanced Water Treatment Methods
- It can be defined as "It is a process of cleaning water to destroy bacteria and all the microorganisms and also to prevent their growth, to make the water safe for consumption.
- After the process of filtration is completed, the water stall can have same impurities like Bacteria, Dissolved inorganic salts colour, odour and taste and iron and manganese.
- So, it is necessary to disinfect the water before it is sent for the consumption through the distribution system. The method of disinfection is different than the method of sterilization.
- In case of disinfection, only the harmful bacteria are killed while in case of sterilization, both harmful and harmless bacteria are killed.
4. 3 Factor affecting disinfection:
- Physical factors:
- The temperature, pH, humidity and hardness of water affect the efficiency of disinfection.
- Several bodily and chemical elements additionally have an impact on disinfectant procedures: temperature, pH, relative humidity, and water hardness.
- For example, the interest of maximum disinfectants will increase because the temperature will increase, however a few exceptions exist. Furthermore, too wonderful a growth in temperature reasons the disinfectant to degrade and weakens its germicidal interest and as a consequence may produce a capability fitness hazard.
- A growth in pH improves the antimicrobial interest of a few disinfectants (e.g., glutaraldehyde, quaternary ammonium compounds) however decreases the antimicrobial interest of others (e.g., phenols, hypochlorites, and iodine).
2. Biological factors:
- The number and location of the microorganisms, their resistance affect the efficiency of disinfection.
- Implicit in all disinfection strategies is the consideration that the most resistant microbial subpopulation controls the sterilization or disinfection time.
- That is, to destroy the most resistant types of microorganisms (i.e., bacterial spores), the user needs to employ exposure times and a concentration of germicide needed to achieve complete destruction. Except for prions, bacterial spores possess the highest innate resistance to chemical germicides, followed by coccidia (e.g., Cryptosporidium), mycobacteria (e.g., M. Tuberculosis), nonlipid or small viruses (e.g., poliovirus, and coxsackievirus), fungi (e.g., Aspergillus, and Candida)
3. Type of disinfectants:
- The concentration and potency of the Disinfectants also affect the efficiency of disinfection.
- The three chemicals most commonly used as primary disinfectants are chlorine, chlorine dioxide and ozone. Monochloramine, usually referred to as chloramine, is used as a residual disinfectant for distribution.
Following disinfectants are commonly used for disinfection of water.
A) Sodium Hypochlorite
B) Chlorine dioxide
C) Peracetic acid
D) Quaternary Ammonium compounds
E) Lodophors
F) Amphoterics
G) Biguanides
Key takeaways:
- The temperature, pH, humidity and hardness of water affect the efficiency of disinfection.
- It is easy to apply due to its high solubility.
- It is available in all forms i.e., Gas, liquid and solid (powder).
- It stops all the metabolic activities are stepped because it is very toxic to most of the micro-organisms.
- The solution left after the chemical reaction, is harmless and still useful to provides in the water distribution system
- The results remain for a very long time.
- This system is cheap and very reliable.
- It is doubtless that for disinfection of water chlorination is one of the best methods.
- The only danger in this treatment is that it needs to have the correct dosage to get the best results. If the dosage is less than the required, it fails to disinfect the water and if it more than the required quantity, it changes the taste and may change odour.
- If the water has excessive amount of organic material, sulphides, nitrite or if the pH of the water is too high or too 1000 i.e., if it is alkaline or acidic. The process of chlorination is affected. It needs correct doses of chlorines.
- Effect of pH chlorination: It has been observed that the Hypoclorite ion (OCI) which formed at the high pH level, is a more effective virucidal form of free chlorine than Hypochlorous acid (HOCI) which occurs at Low pH level.
Methods of chlorination:
- As Chloramines
- It has been observed that chlorine alone is not stable in water but when it is mixed with ammonia, it forms the compounds, which are called as "chloramines".
- They are stable in water and have the capacity to remove the odour upto some extent.
- The ratio of ammonia to chlorine is 0.5: 1 and may change upto 0.25 1. The ammonia dissolves very easily in water but close not diffuse so easily to it needs to have some mechanical means to mix it.
- The ammonia may be used in a form of gas or may as a solution (in the form of Ammonium sulphate or as Ammonium chloride). Before the water is supplied for the consumption, it is necessary to have an interval of about 20 to 60 minutes after adding ammonia to be water.
- This method has a lot of advantages like
- It is more effective
- The effect remains for a long time.
- As it has less irritation to nose and eyes it is mainly used for the disinfection of swimming pools.
- There is no danger of giving lesser or over-dose.
2. As A Free Chlorine Gas
- Either by mixing the gas in small quantity of water and then giving the solution, to the water which is to be treated, or by converting gas into liquid by applying pressure (which is about 0.7 N/mm); the water is disinfected.
This method has following advantages
- It needs less space to store.
- It is very powerful to kill the bacteria.
- It is easy to transport.
- The results of disinfection of water are uniform and reliable.
- Its mechanism is easy and no skilled supervision is required.
3. As bleaching powder
- It is white powder having about 30%, to 35% of active chlorine but it needs to be used with utmost care as it loses the proportion of active chlorine when exposed to atmospheric. It is also necessary to have the laboratory testing of the strength of the powder; to fix the dosage. So, this method is proved to be tedious, laborious and expensive also.
Key takeaways:
- The ratio of ammonia to chlorine is 0.5: 1 and may change upto 0.25 1. The ammonia dissolves very easily in water but close not diffuse so easily to it needs to have some mechanical means to mix it.
- In the Fig. It has been indicated about the status of the residual as the function of the dosage of chlorine.
- From the zero-chlorine applied at the beginning of the abscissa upto the point 'A', the chloride which has been applied is consumed immediately, by reducing such species, such as Fe²+, Mn²*, H₂S and Nitrites.
- In this zone no residual chlorine is produced. From the points between 'A' and 'B' the chlorine reacts with the organic compounds, ammonia and amines and it produces, chloro organic species and also chloramines.
- In this zone no free chlorine is formed.
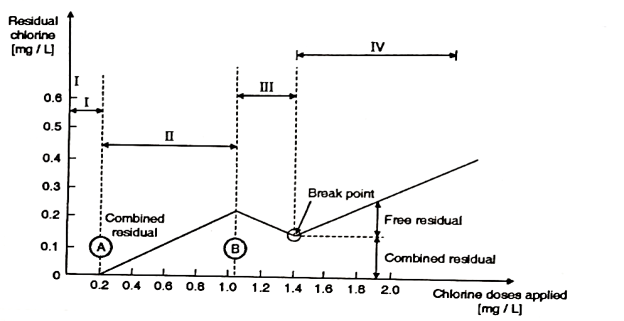
Fig 1: Break Point Chlorination
Zones
I: The Destruction of chlorine Residual; by reducing compound.
II: The formation of chloro-organic compounds and chloramines.
III: The destruction of chloramines and chloro-organic compounds.
IV: The formation of free-chlorine.
- Following chemical reactions control the production of mono and dechloramines.
NH3 + HOCI →→ NH₂CI (Monochloramine) + H₂O.
NH₂CI+ HOCI →→NHCI, (dechloramine) + H₂O.
- The proportion these two forms mainly depends upon the PH and the temperature. From point 'B' to the minimum, which has been designed as the break point, the dischloramine is decomposed to form Nitrogen trichloride as shown below:
NHCI₂+ HOCI →→NCI, (Nitrogen trichloride) + H₂O
- In this range of the chlorine dosage the chloramines also decompose to form N, and N, O. The expected reactions of these decompositions are as shown below:
NH₂CI+ NHCI₂+ HOCI→N2o+4HCl
- So, "The Break Point is a point in the chlorine dose residual curve at which all the destructible chloramines and also the chloro-organic compounds are decomposed and the free residual chlorine begins to appear".
- Beyond this break point the residual chlorine is composed of 'free and combined residual'.
- So, the break point, in the chlorination of water can be defined as, "It is point on the applied residual chlorine curve at which almost all the residual chlorine is a free chlorine".
- The free Residual Chlorine is that part of the residual remaining the water, which will react chemically or biologically as, HOCI or OCI ions.
The advantages of Break-point chlorination
(i) It removes the taste (bad) and odour of water.
(ii) The required residual chlorine remains in the water.
(iii) It helps to remove the organic matter and M
(iv) It helps to complete the oxidation of ammonia and other compounds.
On an average the break point remains between 3 and 7 PPM of chlorine dose. It may get affected by the free ammonia from the water.
Key takeaways:
- "The Break Point is a point in the chlorine dose residual curve at which all the destructible chloramines and also the chloro-organic compounds are decomposed and the free residual chlorine begins to appear".
- Most of the hypochlorites are normally obtained only in the solution but the calcium hypochlorite exists in the solid form in the commercial Bleaching powder.
- It consists of a mixture of calcium hypochlorite and the basic chloride, some free slaked lime is present. The active constituent is hypochlorite.
- It is responsible for the bleaching action, upon the treating bleaching powder with hydrochloric acid.
- The chlorine is liberated, as,
ClO + Cl + 2H = CI₂ + H₂O
- The bleaching powder centerius 36 to 38% of available chlorine. Two methods are used, to determine the available chlorine.
- In the first method hypochlorite solution is treated with excess of a solution of potassium iodide and strongly acidified with acetic acid.
- In the 2nd method, the hypochlorite solution is treated against standard sodium arsenite solution. It is done by adding excess of the arsenite solution then back titrating with the standard iodine solution.
- It is a known fact that if the natural water has the salts if calcium and magnesium, which make the water 'hard'.
- Infact the hard water is not harmful to the human health but when it is used for often purposes like, washing the garments, in dying system, it needs to have more soap, more fuel for boiling and it make the food tasteless. So, it is necessary to treat the hard water to make it 'soft' before it is put the other usages.
Classification of Hardness
- On the basis of the duration of the hardness of water it can be classified as Temporary hardness and Permanent hardness.
- The temporary hardness of water is technically called as carbonate hardness. It is result of the presence of bicarbonates of calcium and magnesium.
- The permanent hardness of water is called as non-carbonate hardness. It is the result of the presence of Sulphates, Chlorides and Nitrates of Calcium and Magnesium.
Demineralization (Removal of hardness of water):
- The temporary hardness of the water can easily be removed either by boiling it or by adding lime. Both, calcium carbonate and magnesium carbonate are not soluble in water, so they easily can be removed in the sedimentation tanks.
- When a large volume of water needs to be treated to remove the hardness, boiling is not practicable. So, the mixing of lime is the best method to remove the temporary hardness of water. Removal of the permanent hardness of water is not easy.
- It needs to have a special treatment, which is called as water softening treatment four different methods can be used to remove the permanent hardness of water.
- In this process lime and soda ash i.e., sodium carbonate is used to remove the hardness of water. The hardness is brought down by 3° to 4°, by this method.
- The equipment’s necessary for this process are:
- Feeding and mixing apparatus
- Settling tank
- Recarbonation plant
- Filters
- In this process, following steps are taken:
- In addition to lime and soda ash, the aluminium compounds are mixed with the water.
- If necessary, some more lime is added which precipitates magnesium.
- The next step is to neutralize the extra lime added in the water by the action of carbon dioxide. It is called as Recarbonation.
- A part of the water under treatment is taken separately and is given as excessive treatment and this water brought to the lowest level of hardness is mixed with the rest of the raw water to remove the total hardness.
- This split method saves time and also the chemicals and reduces the cost of removal of hardness of water.
- It can remove hardness upto 50 mg per lit. So, it does not provide zero hardness.
Advantages of lime soda process:
- The pH value of the treated water gets increased, so the problem of corrosion is reduced.
- The need of more quantity of coagulant is reduced.
- To some extent, it helps to remove the Iron and manganese.
- It also helps to kill some pathogenic bacteria.
- It is a cheap method.
- It is the most suitable method for turbid and acidic waters.
- It is a very simple method and can be added to any water filtration plants.
- Lime is added along with Alum is jar test.
- The jar test is common laboratory test procedure used to determine the optimum dose of different coagulants on a small scale in order to predict the functioning of a large-scale treatment operating conditions per water or waste under treatment.
- In this test the lime is added along with Alum to produce microfloc to make them settle under the gravity.
Disadvantages of lime soda process:
- It needs to dispose a very large quantity of sludge.
- It needs an accurate dosage of chemicals to get the best results, so it needs to have skilled supervision.
- If the recarbonation is not carried, the pipes of the distribution system may get blocked by the thick layers of calcium carbonate.
- As the depth of the filter increases, the capacity of filters increases. The bed of zeolite has either upward or downward flow. In case of downward flow type bed, the hard water is admitted from the top zeolite layer; then it passes through the gravel layer. By using the under-drainage system, the softened water is collected. The rate of flow is about 6000 lit; per hour, per m² of the filter area.
Key takeaways:
- When a large volume of water needs to be treated to remove the hardness, boiling is not practicable. So, the mixing of lime is the best method to remove the temporary hardness of water. Removal of the permanent hardness of water is not easy.
As already discussed, there are two types of hardness of water.
A: The Temporary hardness or carbonate hardness
B: The Permanent hardness Non-Carbonate or hardness
A: In case of Carbonate hardness, which is caused by
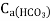 ,
, 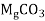
- The Calcium carbonate hardness is in the form of Calcium bicarbonate. It is removed by precipitating it in the form of Calcium carbonate (CaCO3).
Ca (HCO3)₂ + Ca (OH)₂ → 2 CaCO,↓ + 2H₂O
Carbonate hardness of magnesium is in the form of Mg
(HCO3), which is precipitated in the form of Mg (OH)₂.
Mg (HCO3)₂ + 2 Ca (OH)₂ → Mg (OH)₂ CaCO₂+ + 2H₂O
- Finally, the magnesium ion is paired with CI, SO and NO, this hardness can be removed by adding soda ash or also by adding the combination of soda ash and lime. In such case lime will precipitate Mg in the form of Mg (OH)₂. It also produces calcium non-carbonate hardness (CaSO, or CaCl₂). For this, to precipitate the calcium ions the soda ash is added.
The chemical reactions are as follows
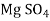 +
+ 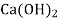 → Mg (OH)₂ ↓ +
→ Mg (OH)₂ ↓ + 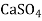
- CaCl₂ +
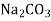 →
→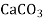 ↓ + 2
↓ + 2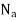 Cl
Cl - It is necessary to note down that while the water is to be softened, contains CO₂. So, when the lime is added into the water, it reacts with CO₂ by consuming more lime;
CO₂ + Ca (OH)₂ → CaCO3↓ + H₂O
- All the natural waters have various concentrations, dissolved salts which dissociate in water to form charged ions. The positively charged ions are called cations while negatively charged ions are called Anions.
- The ions impurities can affect the efficiency and reliability of a boiler or a process system. The hardness ions, like, calcium, and magnesium, must be removed from the water, before it is used as a boiler feed water.
- Ion exchangers exchange one ion for another and hold it temporarily and then release it to a regenerant solution.
- In an ion exchange system, the undesirable ions in the water are replaced with more acceptable ions e.g. In a sodium zeolite, softener, scale-forming calcium and magnesium ions are replaced with sodium ions.
Classification of lon-exchange Resins
Ionizable groups attached to the Resin head. Determine, the functional capability of the region. The industrial water treatment resins are classified into four categories as,
A: Strong Acid Cation (SHC)
B: Weak Acid Cation (WAC)
C: Strong Base Anion (SBA) D: Weak Base Anion (WBA)
Before we talk about the process of reverse osmosis, we must know what is the process of osmosis
(1) Process of Osmosis
- It is natural process of fluid flow across the semi permeable membrane barrier.
- As shown in a tank one side has pure water and other side has salty concentrate solution.
- Due to change in pressure and temperature the diluted solution would move towards the concentrated solution to reduce the salts.
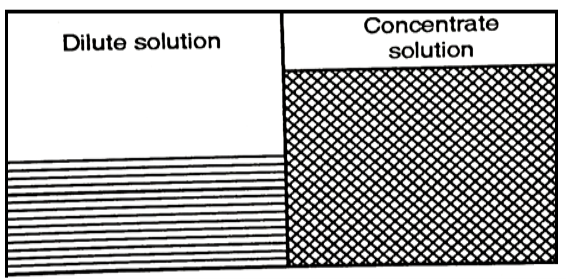
Fig 2: Process of Osmosis
(2) Process of Reverse Osmosis
- If an external pressure to the salt concentration solution is provided the equilibrium would be developed.
- This additional pressure will raise the chemical potential of the water in the salt solution and will cause a solvent flow to the pure water side as it has a lesser chemical potential. This process is called as Reverse Osmosis.
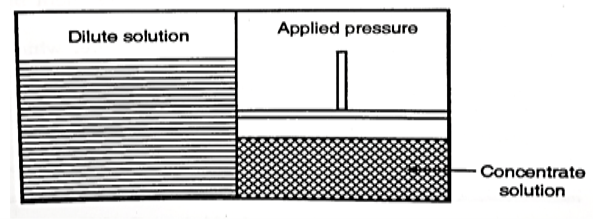
Fig 3: Process of Reverse Osmosis
Key takeaways:
- Due to change in pressure and temperature the diluted solution would move towards the concentrated solution to reduce the salts.
- The rain water which flows over and through the soil, sand gravels etc. Acquires Iron in additions to other minerals.
- The proportion of iron acquired by the surface or ground water depends upon the formation of the rock.
- Manganese may in small proportion to iron also is acquired by the running and ground water. When the proportion of iron and manganese is more than 0.30 P.P.M.
- It is necessary to remove them due to following reasons.
a) When such water is used for washing the clothes, it creates the reddish or brownish spots on the cloths. If it is used in textile industries it develops the same stains on the cloth.
b) When such water is allowed to pass through the pipes, it blocks the mains.
c) The water becomes unpleasant in taste.
d) When the water is to be used in the industries such as paper and pulp, photographic film manufacturing ice making etc. It is necessary to make such water totally tree from iron and manganese.
- It has been observed that if the fluoride concentration is about 1 p.p.m. In the drinking water, it causes reduction in cavities of teeth among the young children. It also helps to reduce the decaying and missing of teeth.
- Generally, in a public water supplies, the fluorides compounds which are used
A: Sodium Fluoride (NAF)
B: Sodium Hexa fluorosilicate (Na₂Si F6)
C: Hexa Fluorosilicic (H₂ Si F6)
- The fluorides which are naturally available in the water are cryolite (Al F3 Na F), Fluorspar of Calcium Fluoride (Ca F₂) and Fluorapatite (3Ca (PO4)₂ CaF₂)
Sources of fluorides:
- It has been observed that the Cryolite and Fluorspar are generally found in the Volcanic areas. Fluorapatite is associated with the phosphate deposits.
- The reaction of these fluorides with the teeth enamel is ionic and so any fluoride compound, which gives ionized fluoride in a diluted solution gives good results.
- It should be noted that there is no difference between the fluorides naturally available in the water and the fluorides which are artificially added in the drinking water both of them give same results.
- Just like chlorine, the water does not have any fluoride demand and so, whatever fluoride is added in the water, it appears in the distribution system. The purpose of chlorination is different than Fluoridation. Chlorination is used to treat the water while fluoridation is carried to improve the physical comfort with respect to dental cavities of the people who consume such water.
Application of Fluorides
- The application of fluorides in the drinking water can be either in the solution form or in a powder form. This mainly depends upon the characteristics of the fluoride compound to be used for the process of fluoridation.
- The feeding fluorides in the water is done separately and not along with chlorine. The excess fluorides are removed by the method of defluoridation.
Feeding of fluorides:
- It is a commercial product which is available in the form of a white, free flowing, non-hygroscopic, crystalline powder having 98.8% purity and has about 59.7 % fluorides.
- It has a very poor solubility in water. The concentration of a saturated solution varies between 0.43 and 0.77 % at 0°C and 25°C respectively. The PH of this saturated solution is between 3.5 and 4.0.
- As this compound is available in powder form, it has to be fed Gravimetrically or Volumetrically and so, it is suitable to treat a large quantity of water.
- There is always a chance of dust hazards. This solution is corrosive to the metal and also it is harmful to the skin. It is necessary to have violent agitation, to maintain uniform and constant strength.
- It is necessary to use such material for the apparatus which contains this solution to be resistant. It is also necessary to take precautions to see that this solution does not settle in the distribution pipes.
- If in water which is to be consumed has excess amount of fluorides it is necessary to go for defluoridation for the consumption.
- The permissible limit of fluoride in the drinking water is 1 to 2 p.p.m. Above this limit it is harmful to consume this water.
The methods of defluoridation are as discussed below
1) Use of Activated Carbons
- The activated carbon produced from various materials can be used to defluoridate the water.
2) Use of lime-soda process
- For water softening the lime-soda process is used. In this process, along with the removal of the magnesium, the excessive fluorides also are removed.
3) Use of calcium phosphate
- Together with calcium phosphate bone charcoal, synthetic tricalcium phosphate etc. can be used to defluoridate the water
4) Use of filter beds
- The water is allowed to pass through the filter beds, the contains of fluorides can be removed.
- However, all the above methods are very costly and have poor fluoride removal capacity. So, a new technique has been found the remove the excess fluorides. It is called as Nalgonda Technique.
- In this method lime i.e., sodium aluminate, bleaching powder and filter-alum are added in sequence to the water. Then the water is stirred for about ten minutes and is allowed to settle for one hour and without disturbing the sedimented material the water is removed smoothly.
- Chemically, the lime helps to have fast sedimentation, bleaching powder helps to have disinfection. The dose of alum depends upon the concentration of fluorides, the alkalinity and total dissolved solids in the water.
- The above-mentioned technique is easy and economical. It can be used at a family level as well as at the society level on a mass scale.
Key takeaways:
- The application of fluorides in the drinking water can be either in the solution form or in a powder form. This mainly depends upon the characteristics of the fluoride compound to be used for the process of fluoridation.
- CNTs are allotropes of carbon with a cylindrical nanostructure. Depending on their production process, CNTs are classified as single-walled nanotubes and multiwalled nanotubes, respectively. Besides having an excessive unique floor area, CNTs own relatively assessable adsorption web sites and an adjustable floor chemistry.
- Due to their hydrophobic floor, CNTs need to be stabilized in aqueous suspension so that you can keep away from aggregation that reduces the energetic floor. They may be used for adsorption of continual contaminants in addition to preconcentrate and stumble on contaminants. Five Metal ions are adsorbable via way of means of CNTs via electrostatic appeal and chemical bonding. Furthermore, CNTs showcase antimicrobial houses via way of means of inflicting oxidative pressure in microorganism and destroying the mobileular membranes. Although chemical oxidation occurs, no poisonous byproducts are produced, that's a vital benefit over traditional disinfection approaches like chlorination and ozonation.
- They may be surely regenerated via suitable changes of working conditions, like pH shift. Conventional desalination techniques are energy-eating and technically demanding, while adsorption-primarily based totally strategies are easy and smooth to apply for point-of-use water purification gadgets, but their ability to eliminate salts is limited. Yan et al8 evolved plasma-changed ultralong CNTs that characteristic an ultrahigh unique adsorption ability for salt (exceeding 400% via way of means of weight) this is orders of significance better while in comparison with traditional carbon-primarily based totally water remedy systems.
- These ultralong CNTs may be carried out in multifunctional membranes which can be capable of eliminate now no longer simplest salt however additionally natural and metallic contaminants. Next-era potable water purification gadgets prepared with those novel CNTs are anticipated to have advanced desalination, disinfection, and filtration houses.
- Recently, a group of US researchers evolved a sponge product of natural CNTs with a sprint of boron that suggests a fantastic cap potential to take in oil from water.
- The oil may be saved withinside the sponge for later retrieval or burned off so the sponge may be reused. If they achieve producing huge sheets or discover a manner to weld the sheets, the sponge fabric may be carried out in getting rid of oil spills for oil remediation.
- Although CNTs have huge blessings over activated carbon, their use on a commercial scale for huge municipal water and wastewater remedy flowers isn't always anticipated withinside the midterm due to excessive manufacturing costs.10 Point-of-use packages that require small portions of CNTs are extra competitive; for example, for the removal of closely degradable contaminants which includes many antibiotics and pharmaceuticals.
- The methods of desalination are used to remove the salts from the water.
- The application of the processes mentioned below. Depends upon, the salinity of the water to be treated, the end use of the treated water, the economic budget for the process of desalination of water.
Methods of desalination:
- Solvent Extraction
- When the water has low salinity i.e., less than 100 gram/per 1000 grams of water, this method is applied. The organic solvents such as secondary and tertiary amines are mixed with the oceanic water to get more concentrated raffinate and then an extract containing low salinity water gets separated by heating it.
- This solvent can be recycled which helps to reduce the cost of desalination process.
2. Electrodialysis
- The process refers to the transport of ions from the salt solution, through the ion exchange membranes, as a result of an applied electric field.
- It consists of a number of cation and anion exchange membranes which are arranged alternately. The saline water is fed through the manifold in alternate compartments.
- The cations and the anions move towards the cathode and anode, through the cation and anion exchange membranes respectively, leaving back the feed depleted in salt while the neighbouring compartments become concentrated.
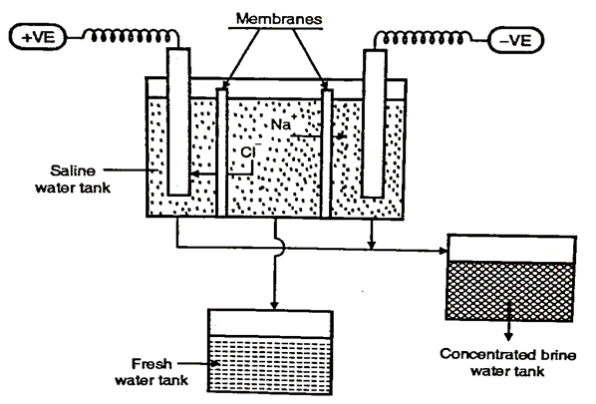
Fig 4: Electrodialysis
- As this method needs to have higher power requirement as the proportion of dissolved solids it is adopted only for the water, having less than 10,000 mg/lit of dissolved solids.
3. Solar Distillation
- In this method the solar energy is used for desalination of saline water.
- The sunrays are allowed to pass through panel of glass having heat insulated back under this glass roof. The saline water is kept in a shallow pan.
- The water gets evaporated and it comes back in a form of freshwater drop which are collected on either side of the saline pan. The rate of distillation depends upon the latitude i.e., angle made by the sunrays and duration of the day.
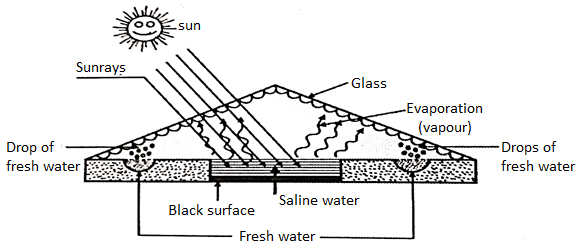
Fig. 5: Solar Distillation
4. Freezing Method
- When the sea water (Saline water) freezes the ice crystals are formed which are free from salts. If these crystals are separated and melted, they can yield fresh water.
- It is a costly method of desalination of saline water because it needs to use a considerable quantity of pure water to wash the salts from the ice crystals.
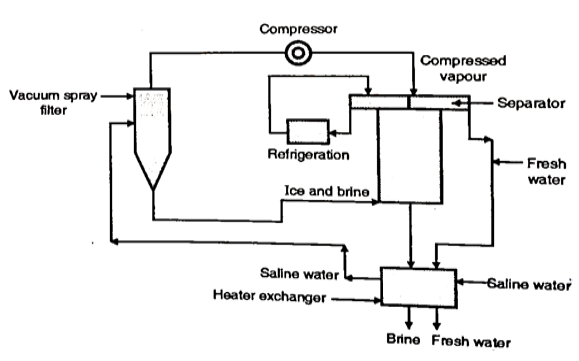
Fig. 6: Freezing Method
Key takeaways:
- The application of the processes mentioned below. Depends upon, the salinity of the water to be treated, the end use of the treated water, the economic budget for the process of desalination of water.
References:
- Environmental Engineering, Peavy and Rowe, McGraw Hill Publication
- Optimal Design of Water Distribution Networks, P.R. Bhave, Narosa Publishing House
- Rain Water Harvesting: Making water Every Body’s business, vcentre for science and Environment
- Environmental Remote sensing from Regional to global Scales, Ed. Giles Foody, Wiley
- Water supply Engineering, Harold FatonBabbit& James Joseph Doland, Tata McGraw Hill
- Environmental Engineering Laboratory Manual and Dr. N. Kumarswamy. NEERI, Nagpur