UNIT 5
Conducting materials
A conductor contain general properties are given below-
In the condition of equilibrium conductor exhibits following properties.
Resistance
Inductance
Electric field inside the conductor is 0
Density of charge inside the conductor is 0
On the conductor surface free charge exists only.
At the conductor surface, electric field is normal to surface.
Resistance
Generally, conductor’s electricity possessed too low resistance for electricity flow. A perfect conductor contain resistance zero ideally. But, in practical conductor’s resistivity varies from low to high. Conductor containing low resistivity or high conductivity are utilized as the conductor winding of the electrical machines, for electrical contact, for transmission lines and earth wires etc. the conducting materials keeping high resistivity or low conductivity are utilized for building heating elements and filaments incandescent lamp for the electric heaters, furnaces and ovens.
Inductance
In AC supply when a conductor is utilized, a magnetic flux is developed. This flux has two parts. Internal and external flux. Internal flux has the very low value as comparison of the external flux. Cause of this flux linkage to the conductor itself and inductance is come into the picture. This occurred inductance give results in extra voltage drop in the conductor. Besides, this inductance effect distribution of the current over the cross-section area of the conductor. Because of, current is always preferring to flow by outer part of the cross-sectional area. And this effect named skin effect. This distribution of the current over the cross-sectional area is affected through flux linkage to the conductor besides to the current by the nearby conductor. This is the proximity effect and both effect proximity and skin effect exist only the for AC supply. Not for the DC supply.
Electric field inside the conductor zero
A perfect conductor has electric field zero. In a conductor, if electric field is exists, it will lead a force on the electron and accelerate them. But, when the equilibrium condition is occurred net force on the electron is zero. Thus, electric field is not exist in conductor. This show electric field must be outside the conductor. This property is used for the electrostatic shielding for the electrical equipment.
Charge density inside the conductor is zero
Inside the conductor electric charge does not exist. The reciprocal electrostatic repulsion force, between charges that is electrons, requests which the electrons should be as far as possible. This force pushes the electrons to surface on the conductor. Besides there is no electric charges exists in conductor outcomes in the zero-charge density into the conductor.
Free charges exist only on conductor surface
As we know that the charge particles do not exist inside conductor. Besides of the electrostatic repulsion force, electrons are move to the outer surface of the conductor. This is happening only by the electric charges which exists inside the conductor. Therefore, free electrons exist only on surface of conductor.
Conductor surface, electric field is normal to surface
When we discuss about the boundary condition of the dielectric to the conductor, electric field is normal to the conductor surface and electric field tangent part to surface is zero. This give the information about the electric field intensity is normal for the conductor surface and electric field tangential part intensity is zero.
Materials which are provide conduction of electricity due to the free electron and an electric potential is difference is applied over them are called as the conducting materials. These materials are good conductor of the electricity and heat. Current and heat is flow due to the free electron in the materials.
Free electron theory
A material keeps a large number of free electrons that are free to transfer about in the complete volume of metal such as molecules of the gas in a vessel.
Free electrons are move in the random direction and run into either +ve ions fixed in other free electrons. There is no energy loss and all electrons are elastics.
The energy distribution of the free electrons follows the classical Maxwell Boltzmann statistics. Free electrons are transferring in the fully uniform possible field besides the ions which are fixed in other free electrons.
When the electric field is absent free electron random motion is equal probable in all the directions therefore, the current density vector is 0.
When we applied external electric field is applied over ends of metal, electrons drift slow with some average velocity named as drift velocity in opposite direction to electric field. Drift velocity is the superimposed across random velocity. This is responsible for electric current flow in the metal.
Electrical conductivity
Electrical conductivity is described as the charge rate flow over unit area in the conductor per unit potential voltage gradient.
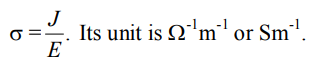
5.2.1 Aluminum
Aluminum is electrically conductive material. Which is discovered by Hans Oersted in 1825. The name aluminum is derived from the Latin language for alum, alumen meaning bitter salt. Its atomic number is 13. Electron configuration is [Ne]3s23p1. Aluminum is the silvrey white and light weight metal. It is the malleable and soft. Properties of the aluminum are
Strength to weight ratio
Corrosion resistance of aluminum
Thermal and electrical conductivity of the aluminum
Heat and light reflectivity of aluminum
Aluminum toxicity
Recyclability of aluminum
Production of aluminum
Aluminum smelting
Environmental considerations
Applications
Different type of aluminum alloys has provided outcome in being aluminum utilized in the industries as the food preparation, diverse as the transport, packaging, energy generation, electrical transmission and architecture applications. Aluminum material is replaced naothe materials like steel, zinc, tin plate, stainless steel, copper, wood , paper, composites and concrete. These all applications takes approximately 85% of aluminum hold annually. 15% which are left is utilized in the applications including- high pressure gas, cylinders, ladders, sporting goods, road barriers and signs, furniture, lithographic printing and plates.
5.2.2 Copper
The term copper is derived from the latin word ‘cuprum’, that means ‘ore of the cyprus’. So the symbol of the copper is Cu. The material copper is tough, malleable and ductile materials. Having these properties make the copper extremely suitable for the wire drawing, deep drawing, tube forming and spinning. The other properties are
Excellent heat conductivity
Electrical conductivity
Excellent carrion resistance
Better biofouling resistance
Better machinability
Mechanical and electrical properties retention at the cryogenic temperatures.
Non magnetic
Copper has the melting point is 1083 degree C.
Applications are
Copper material permits heat to transfer through it fast. It is so utilized in more applications where fast heat transfer is essential. In this include-
Device | Use |
Copper plate | Sauce pan bottom |
Copper pipes | In hot water tanks heat exchangers, all weather football pitches, under floor heating system and car radiators. |
Heat sinks | Disk drives, TV sets and computers. |
Resistivity
Semiconductors resistivity is strongly relying on presence of the impurities in materials, a fact that makes them too useful in the state of solid electronics. Conductor electrical resistance of the unit cross sectional area and length. Each material characteristics property, resistivity is useful for comparing different materials on their ability to conduct the electrical currents. Poor conductors have the high resistivity. Resistivity table is given below.
Material | Resistivity ρ (ohm m) | Temperature coefficient α per degree C
| Conductivity σ x 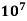
| Ref | |
Silver | 1.59 | 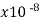 | .0038 | 6.29 | 3 |
Copper | 1.68 | 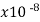 | .00386 | 5.95 | 3 |
Copper, annealed | 1.72 | 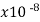 | .00393 | 5.81 | 2 |
Aluminum | 2.65 | 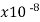 | .00429 | 3.77 | 1 |
Tungsten | 5.6 | 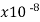 | .0045 | 1.79 | 1 |
Iron | 9.71 | 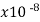 | .00651 | 1.03 | 1 |
Platinum | 10.6 | 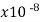 | .003927 | 0.943 | 1 |
Manganin | 48.2 | 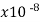 | .000002 | 0.207 | 1 |
Lead | 22 | 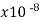 | …. | 0.45 | 1 |
Mercury | 98 | 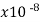 | .0009 | 0.10 | 1 |
Nichrome (Ni, Fe, Cr) | 100 | 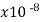 | .0004 | 0.10 | 1 |
Constantan | 49 | 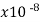 | … | 0.20 | 1 |
Carbon* (graphite) | 3-60 | 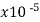 | -.0005 | … | 1 |
Germanium* | 1-500 | 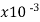 | -.05 | …. | 1 |
Silicon* | 0.1-60 | … | -.07 | … | 1 |
Glass | 1-10000 | 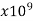 | …. | …. | 1 |
Quartz (fused) | 7.5 | 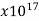 | …. | …. | 1 |
Hard rubber | 1-100 | 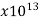 | … | …. | 1 |
5.3.1 Nickel-chromium alloy
Formula – Ni/Cr
Nickel-chromium alloy describes that chromium is soluble in nickel. That is maximum at the 47% at eutectic temperature and drops off about 30% room temperature. This solution provides the range of the commercial nickel chromium alloys. This nickel chromium alloy has good resistance to the higher temperature oxidation, corrosion and better wear resistance.
Properties of the environmental
Poor=1, excellent =5 | |
Flammability | 5 |
Fresh Water | 5 |
Organic Solvents | 5 |
Oxidation at 500C | 5 |
Sea Water | 5 |
Strong Acid | 5 |
UV | 5 |
Wear | 5 |
Weak Acid | 5 |
Weak Alkalis | 5 |
A normal nichrome alloy is the 80% nickel and 20% chromium, through mass, however many another combination of the metal for different applications. Nichrome is silvery grey in color, corrosion resistant and keeps high melting point of 1400 degree C (2550 degree F).
Physical properties
Lump – 80/20
Piece – 70/30
Targets – 60/40
Powder – 50/50
Chemical properties
31xx | Ni 1.25%, Cr 0.65% or 0.80% |
32xx | Ni 1.25%, Cr 1.07% |
33xx | Ni 3.50%, Cr 1.50% or 1.57% |
34xx | Ni 3.00%, Cr 0.77% |
|
|
|
|
Applications are
a) Oxidation Resistance
b) Heating Elements
c) Thermocouples
d) High Temperature Corrosion Resistant Alloys
e) Wear Resistant Alloys
5.3.2 Tungsten
Tungsten is element with symbol W and atomic number is 74. Tungsten comes from former Swedish name for tungstate mineral scheelite, tungsten means heavy stone. It is found rarely metal naturally on the earth which exclusively merged with another components in the chemical compounds rather than free from. It was recognized in form of new element in 1781 and 1st isolated as metal in the 1783. It is essential ores involve wolframite and scheelite.
Applications are medical and industrial radiation shielding, weights, counterbalances and ballasts, boring bars and grinding quills, bucking bar, ordinance components and high temperature tooling.
Applications
Its primary application for across 100 years have been the filament in the incandescent light bulb. Doped with the little amount of the potassium-aluminium silicate, tungsten powder is impurity at the high temperature to generate wire filament which is n center of the light bulb which light millions of the homes of the around world .
5.3.3 Kanthal
Kanthal alloy is the trademark of the family of the iron-chromium-aluminium (FeCrAl) alloys are utilized in a broad range of the resistance and the higher temperature applications. Kanthal alloys have of the mainly iron, chromium (20-30 %) and aluminium has the (4-7.5%).
Characteristics
For heating and resistance wire should be stable in the air when the hot. Kanthal FeCrAl alloy develops a layer for protection of alumina. Aluminium oxide has higher thermal conductivity however is electrical insulator, therefore specific techniques may needed to develop good electrical connection.
Uses
This alloy is utilized in the heating elements besides to their durability, flexibility and tensile strength. It utilizes are widespread, toasters, industrial heaters and home, diffusion and kilns.
Currently, kanthal has been utilized for the heating coils in the electronic cigarettes.
5.3.4 Silver and silver alloys
Sliver is white metal, which is soft and usually finds in the nature one of four forms:
a) In the form of native element
b) In the form of primary constituent in silver minerals
c) In the form of natural alloys with another metals
d) In the form of trace to the minor constituent in ores of another metals.
Mostly silver which is produced at present is fourth type and product are made from this.
Types of the silver alloys are-
Different types of silver alloys are including Britannia silver, sterling silver , shibuichi and electrum etc.
Utilization of common silver alloys
Normally silver alloys are utilized in making the jewelry. Sterling silver (92.5 % silver and 7.5 % copper) and Britannia silver ahs the (95.84 % silver and 4.16 % copper) are cheaper when compare with the platinum or gold. These are also utilized to make table ware and are also utilized as the currency in many nations.
Copper characteristics
Copper materials are ductile, tough and malleable. These qualities make copper suitable for wire drawing, tube forming, deep drawing and spinning. There are other key properties by copper and copper alloys include:
- Heat conductivity exllency
- Best electrical conductivity
- Better corrosion resistance
- Good biofouling resistance
- Better machinability
- Mechanical and electrical retention qualities at the cryogenic temperature
- Non- magnetic
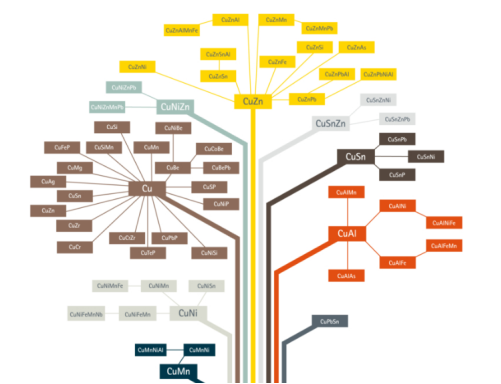
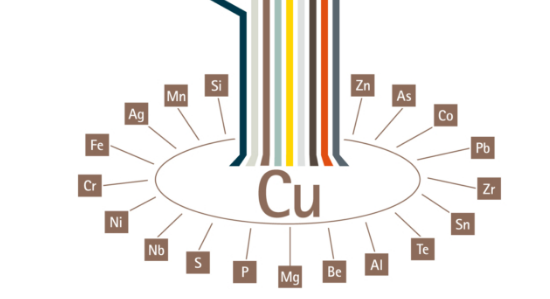
There are many copper alloys are so we represent through a copper tree. Approximate 400 various types of copper and copper alloy constitutions loosely categorized such as high copper alloy, copper, brasses, copper nickels, bronzes, copper-nickel- zinc, special alloys and leaded copper.
Characteristics of bronzes
Copper alloy bronze generally is a golden hard and brittle metal. Due to specific composition of the copper alloy properties depends on. There are some typical characteristics discussed below.
Highly ductile
Bronze shows low friction against the other metals.
Many of the bronze alloys are showing the unusual property of expansion a small amount while solidifying from the liquid into the solid. Like sculpture casting, and helps to fill mold.
Less than brittle from cast iron.
Upon the exposure to the air, bronze oxidizes, however on their outer layer. This patina keeps of the copper oxide, that eventually becomes copper carbonate. The layer of oxides saves interior metal from the further corrosion. But, if the chlorides are presents copper chlorides form, that can be cause bronze disease, a situation in which the corrosion works by metal and damage it.
Far from steel, striking against bronze a hard surface won’t be produce sparks. This develops bronzes useful for metal utilized around explosive materials.
Characteristics of the brass
Copper alloy brass, and zinc of the enduring and historical essential because of their workability and hardness. The premature brass, named calamine brass, dates to the Neolithic times, it was likely build through the reduction of the mixtures of the zinc ores and the copper ores.
The ductility of the brass relies on zinc content, brasses which contain more than 45 % zinc are not performed, either cold or hot. Like brasses, which is known brasses are of the little important on the behalf industry use. This are utilized in soldering, they also make the basis for determine alloys utilized in the die-casting. The soft brasses further subdivided into those which can be performed cold and those with the good zinc content , that need hot working.
These materials are made from graphite and other carbon forms. Electrical machines brushes are making like DC machines and alternators. Parts of the telecommunication equipment are made by these carbon materials. In electrical engineering carbon is used in various forms and in the form of other materials combination. These materials are made from the graphite and other carbon forms. Carbon is keeping following applications in the electrical engineering-
Filament of the incandescent lamp
For developing electrical contacts
Resistors are made from them
Brushes are made from electrical machines like alternators and DC machines. Battery cells are made from them, for developing carbon electrodes for the electric furnaces, welding and arc lighting electrodes, for developing elements for the vacuum valves and tubes, telecommunication equipment’s.
Filament is an essential part of the incandescent lamp. Duration of the incandescent lamp, relies on the filament. Various kind of materials are utilized for making the filament of incandescent lamp. There are some materials are used in filament given below-
Carbon
Tantalum
Tungsten
Solders
Solders are melted when give them to heat from an iron connected to the temperature controller. Solders are heated up to the temperatures exceeds its melting points at the around of 600 degree F that then caused it to melt, that cools making the joints. Materials are used for making solders are- filler metals utilized in the soldering were once lead solder, because of regulations, lead based solders are replaced by the lead-free solders, that may have antimony, brass, copper, bismuth, tin, indium or the silver.
Bimetal or thermocouple metal is a strip of 2 or more composite materials keeping various coefficient of the linear thermal expansions bond through riveting, welding or brazing. Bimetal consists the alloys iron, manganese or chrome and nickel in different type of chemical compositions.
Thermocouple
Thermocouple is electrical device which consisting of the two dissimilar electrical conductors which making an electrical junction. It produces a voltage which dependent on temperature as the results of thermoelectric effect and voltage can be explained of measurement of the temperature. Alloys of the thermocouple alumel utilized in the conjunction with the chromel in the type K thermocouple, alumel 1 is made from the manganese, aluminum, nickel and silicon, rhenium-tungsten, constantan, and chromel utilized with alumel in the type of k thermocouples and with the constantan type E thermocouples, chromel 2 is made of the chromium and nickel.
Reference books
Padiyar, K. (1999). Analysis of subsynchronous resonance in power systems. Boston: Kluwer Academic Publishers.
Sih, G. (2007). Multiscaling in Molecular and Continuum Mechanics: Interaction of Time and Size from Macro to Nano. Dordrecht: Springer.