UNIT1
Crystal Structure and Deformation of Materials
Atom arrangement in material: -
Depending upon the manner of atomic grouping, materials are classified as molecular structure, crystal structure and amorphous (irregular structure).
Molecular structure: This structure has a number of atoms that are held together by primary bond. E.g.: - 
Crystal structure: - In glass the atoms are not regular. This is called amorphous structure.
Unit cell: - A unit cell is defined as the basic structure of atoms arranged in a material. Different forms of unit cell have different length, different angles as given below:
(1) Cubic structure: - a = b = c
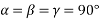
a = length of the unit cell along x axis
b = length of the unit cell along y axis
c = length of the unit cell along Z axis
(1) Tetragonal structure: - a = b ≠c: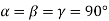
(2) Orthometric: - a ≠b ≠ c: 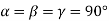
(3) Monoclinic: - a ≠ b ≠ c: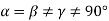
(4) Triclinic: - a ≠b ≠ c: 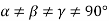
(5) Rhombohedral: - a = b = c: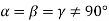
(6) Hexagonal: - a = b ≠ c: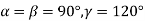
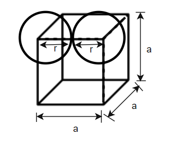
(2) Simple cubic (SC): - Atoms are arranged at the corner of the cell.
Total number of atoms = 8
Number of atoms at each corner 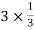
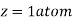
Edge length a = 2r
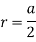
Packing efficiency: - volume of spaces occupied by the space
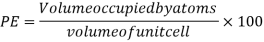
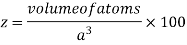
Where z = number of atoms per unit cell
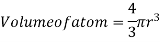
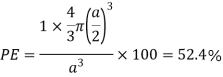
Total space occupied by spares =52.4
Empty space = 100 – 52.4 = 47.6%
(3) Body centered cubic (BCC); - atoms are arranged at the corners of the cube with other atoms at the cubic center.
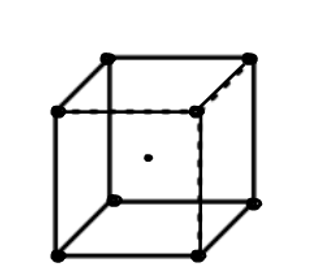
Number of atoms in corner = 8
Number of atoms in each corner 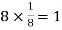
Number of atoms in middle =1
Total atoms = z = 1 + 1 = 2
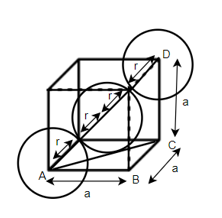
According to Pythagoras theorem
In ∆ADC
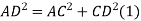
Where AD = 4r
CD = a
AC =?
So, ∆ABC
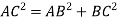
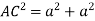
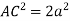
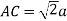
Putting the values in equation (1)
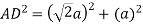
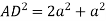
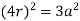
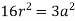
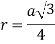
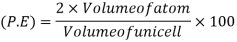
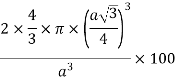
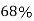
So, volume occupied by the space = 68%
Empty space =32%
Example: - Mg, Na, K, Li
(4) FCC (Face center of cubic): - Atoms are arranged at the corners of each cube for the cell.
Total number of atoms in corner = 8
Each atom in corner 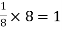
Atoms in each face 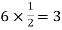
So total atoms in cell = 1 + 3 = 4
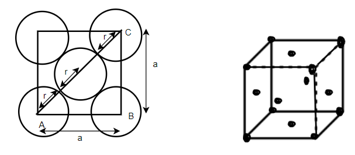
In ∆ABC
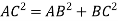
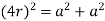
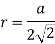
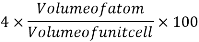
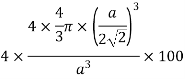
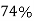
Volume occupied by the space = 7 empty space = 26
Example: - Al, Ag etc.
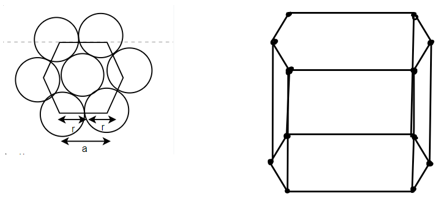
(4) HCP (hexagonal closed packed)
Cell on a hexagon closed packed lattice is visualized at top 4 bottoms plane of atoms forming a regular hexagon around a central atom.
There are two lattice parameters in hexagonal close-packed, a and c representing the basal 4 height parameters respectively.
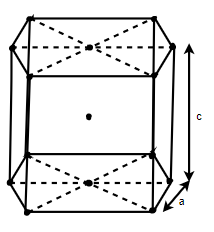
Number of atoms in corners = 12
Number of atoms in each corner = 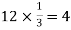
Number of atoms in mid = 01
Number of atoms in top 4 bottom face 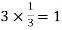
Total atoms = 4+01+01=06
Relation between a and r: -
In hexagonal close packed structure, the atom is in contact with each other along the cube edge.
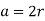
Volume of unit cell: - area of hexagon × h
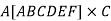
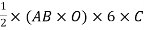
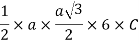
Now, 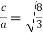
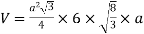
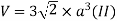
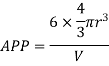
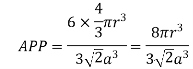
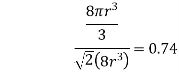
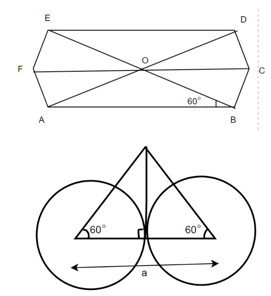
Proof: - c/a
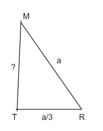
Consider three body atoms PQR
A median is drawn from r to PQ which meets at point S
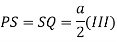
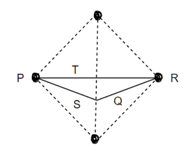
PQR is equilateral triangle.
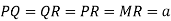
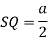
RSQ is a right-angled triangle
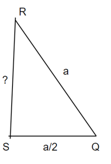
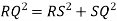
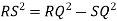
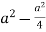
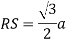
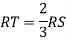
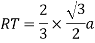
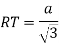
MTR is right angled triangle
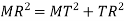
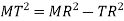
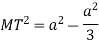
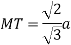
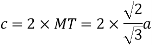
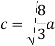
Lattice
A lattice is a par in (L, S) in which every {a, b} consisting of two elements has at least upper bound and a greatest lower band.
LUB {a, b} is denoted by 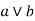 is called the join of a and b.
is called the join of a and b.
aLB {a, b} is denoted by 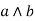 is called the meet of a and b.
is called the meet of a and b.
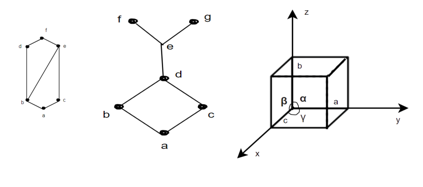
Diagram (a) is lattice.
Diagram (b) is not a lattice as feg doesn't exist.
Lattice Parameters: -
It refers to the physical dimension of unit cell in a crystal lattice. In 3- dimension there are generally three lattice constants referred to as a, b, c (in diagram (c)).
A group of lattice constant code referred to as lattice parameters however the full set of 3 lattice constant three angles between them.
Example: - The lattice constant for diamond is at 300 k.
The structure is equal and but still its actual shape cannot be determined from only lattice constant.
Properties of lattice: -
Idempotent lattice property: - a) 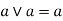
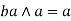
Commutative property: - a) 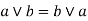
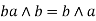
Associative property: -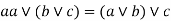
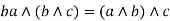
Absorption property: - a) 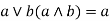
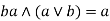
Miller Indices: = Identifying a particular plane or surface in a 3-dimensional crystal using set of numbers.
Procedure: -
(1)
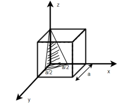
(x, y, z) =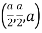
(x, y, z) pu=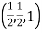
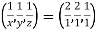
Remove common factor or common number in the denominator then we get Miller Indices.
Therefore, miller indices are (2,2,1)
(2)
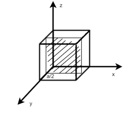
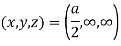
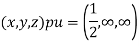
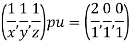
Hence Miller indices = (2, 0, 0)
(3)
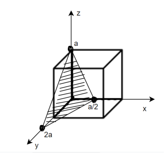
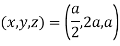
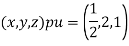
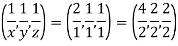
Miller indices = (4, 1, 2)
(4)
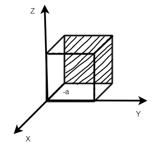
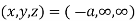
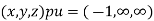
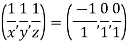
Miller indices = (-1, 0, 0)
Crystal imperfections: - Crystal imperfections are the defects in the regular geometrical arrangement of the atom in a crystalline solid.
The defects may lead to the crystal the formation or else rapid cooling from higher temperature or high energy radiation striking the solid.
these defects directly impact on its mechanical, electrical and optical behaviors of the crystal.
Different types of defects in single atomic crystalline structure: -
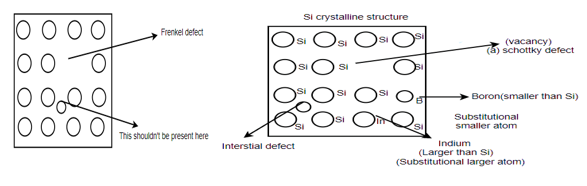
Schottky defect: - A vacancy or vacancies in an ionic crystal structures are called Schottky defect.
Interstitial defect: - atoms that occupy a site in the crystal structure at which there is usually not an atom.
Frenkel defect: - nearby peer of vacancy and an interstitial is called as Frenkel defect.
Substitutional defect: - if different atoms are occupying the locations of the regular atom then they are called substitutional defect.
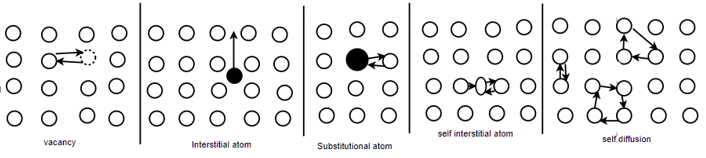
Diffusion mechanism: -
In pure metals self-diffusion occurs where there is no net mass transport, but atoms migrate in a random manner throughout the crystal.
The most energetically favorable process involves and interchange of places by an atom and a neighboring vacancy - vacancy diffusion. This process demands not only the motion of vacancies, but also the presence of vacancies. The unit step in vacancy diffusion is an atom breaks its bonds and jump into neighboring vacant site. In interstitial diffusion, solute atoms which are small enough to occupy interstitial sites defuse by jumping from one interstitial site to another. the unit step hair involves jump off the diffusing atom from one interstitial site to a neighboring site. Substitutional diffusion generally proceeds by the vacancy mechanism. Thus, interstitial diffusion is faster than the substitutional diffusion by the vacancy mechanism.
Self-diffusion or Ring mechanism for direct exchange mechanism, hair three or four atoms in the form of a ring move simultaneously round the ring there by interchanging their positions.
A self-interstitial is more mobile than a vacancy as only small activation energy is required to move to an equilibrium atomic position and simultaneously displays the neighboring atom into an interstitial site.
A) Magnetic property: - The magnetic properties of a material are those which determines the ability of a material to be suitable for a particular magnetic application.
Some different types of magnetic properties are described below
Permeability: - It is the property of magnetic material which indicates that how easily the magnetic flux is built up in the material. Some time it is also called as the magnetic susceptibility of material.
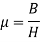
B = magnetic flux density in material in 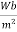
H = magnetizing force of magnetic flux intensity in Wb/ Henry meter
SI unit of magnetic permeability is Henery/meter.
Permeability of material is also defined as
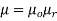
Where, 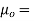 permeability of air or vacuum and
permeability of air or vacuum and
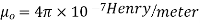
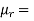 relative permeability of material
relative permeability of material
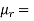 1 for air or vacuum
1 for air or vacuum
NOTE: - A material selected for magnetic work in electrical mechanism should have high permeability so that required magnetic flux can be produced in by less ampere volts.
Retentivity on magnetic hysteresis: -
When magnetic material is placed in an external magnetic field, its grains get oriented in the direction of magnetic field which result in the magnetization of material in the direction of external magnetic field. Now, even after removal of external magnetic field some magnetization exists which is called residual magnetism. The property of material is called magnetic retentivity of material.
A hysteresis loop or B.H curve of a typical magnetic material is in below figure. Br in below hysteresis loop represents the residual magnetism of material.
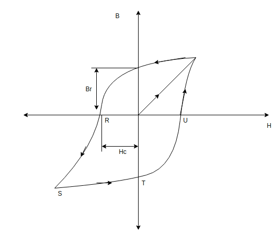
Coercive force: - Due to retentivity of material even after removal of external magnetic field some magnetization exist in material. This magnetization is called residual magnetism of material. To remove this residue all magnetization we have to apply some external magnetic field in opposite direction. The external magnetic motive force (ATS) required to overcome the residual magnetism is called “Coercive force.”
Note: - Material having large value of residual magnetization and coercive force are called magnetically hard materials. the materials having very low value of residual magnetization for coercive force are called magnetically.
Reluctance: - It is a property of magnetic material which results to build up of magnetic flux in materials. It is denoted by R.
Its unit is ampere turns/Wb.
Reluctance = 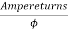
Hard magnetic materials suitable for the electrical core of electrical machines should have low reluctance.
B) Mechanical properties
the mechanical properties of materials are those which affect the mechanical strength and ability of a material to be molded in desired shape.
Some of them are as follows: -
1) Strength
2) Hardness
3) Toughness
4) Malleability
5) Hardenability
6) Brittleness
7) Ductility
8) Creep and slip
9) Resilience
10) Fatigue
C) Optical properties: -; Optical property of a material is defined as its interaction with electromagnetic radiation in the visible.
Optical materials are classified on the basis of their interaction with visible-light into three categories
1) Optical properties – Metals: -
i) Metals consist partially filled high energy conduction bands.
ii) When photons are direction at metals their energy is used to excite electrons into an occupied state. Thus, metal is opaque to the visible light.
iii) Metals are however transparent to high and frequencies i.e. X-rays and Y – rays.
2) Optical properties: - Nonmetals: -
i) Refraction: - when light photos are transmitted through a material, they cause polarization of the electrons and in turn the speed of light is reduced and beam of light changes direction.
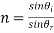
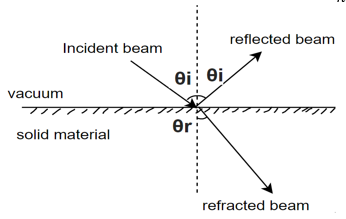
Snell's law of light reflection
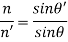
Reflection: - Reflectivity is defined as fraction of light reflected at an interface
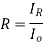
If the material is in the other material with refractive index 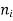
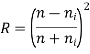
Absorption: - When a light beam is impinged on the incident beam that is not reflected by the material is either absorbed or transmitted through the material.
Optical applications: -
1) Luminescence
2) Lasers
3) Thermal emission
4) Photoconductivity
5) Optical fibers
D) Electrical properties: - The electrical properties of a material are those which determine ability of a material to be suitable for a particular electrical engineering.
Some of them are as follows: -
3) Resistivity
4) Conductivity
5) Temperature coefficient of resistance
6) Permittivity
7) Thermal electricity
8) Dielectric strength
Resistivity: - Properly of a material which resists the flow of electrical current through material. It is the reciprocal of conductivity.
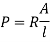
S.I unit = Ωm
Where, R = resistance of conductor in Ω
A = cross sectional area
l = length of the conductor in 'm'.
Conductivity: -
It is the property of a material which allows the flow of current. It is denoted by 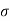
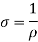
S.I unit = 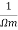
Dielectric Strength: - Property of a material which indicates the ability of material to withstand at high voltages.
S.I. unit = KV/cm
Temperature coefficient of resistance: -
Property of a material which indicates the change in resistance of material with change in temperature.
It is denoted by 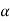
Thermoelectricity: - It is the function formed by joining of two metals when heated, a small voltage in the range milli volt is product. This effect is called thermal electricity.
The change in dimensions in form of better under the action of applied force is called deformation.
Types of metal deformation: -
1) Elastic deformation
2) Plastic deformation
Elastic deformation: - It is the deformations which appears when the load is removed. It happens before the plastic deformation. This information occurs when the stresses applied on a metal piece.
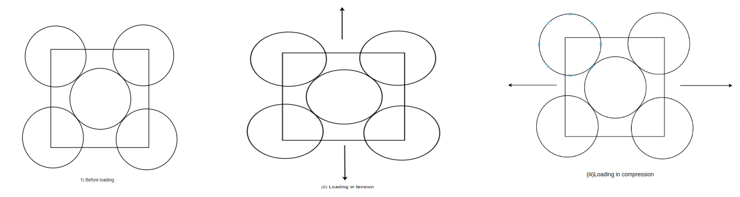
Above figure shows form of the atoms before loading, loading in tension and compression respectively. When a tensile load is applied the piece becomes slightly larger whereas compression load shortens the piece.
For elastic deformation the strain is nearly proportional to the stress.
The ratio between stress and strain and elastic deformation is known as modulus of elasticity or young modulus.
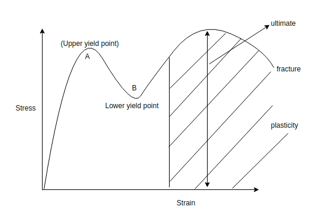
Plastic deformation: - it is the deformation which persist even after the load is removed. Plastic deformation is observed at stresses exceeding the elastic limit which depends primarily on stress in the simplest curves. Plastic deformation is typically a function of stress temperature and rate of straining.
Plastic deformation process is generally applied in metallurgical operation of shaping the operation include rolling of boilerplates, drawing of wire, extrusion of telephone cable etc.
In metal the plastic deformation generally takes place by the process of slipping.
Slip: - It is defined as the shear deformation that moves atom by many interatomic distance in in one crystal planes over the atom of another crystal plane.
Slip process under the share load (because of plastic deformation) crystal is divided into layer of slip blocks which are displaced in reference to each other and are separated by the layer.
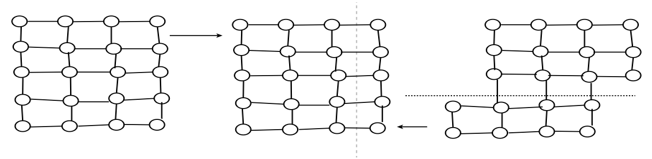
Twinning: - twinning is that process by which a portion of the crystal takes up and orientation which makes that portion a mirror image of the parent crystal.
It is of two types:
a) Mechanical twins: -; It is produced by mechanical deformation. These are produced in BCC or HCP metals under conditions of rapid rate of loading and increases temp.
b) Annealing Twins: - These our product as a result of annealing following plastic deformation.
Work hardening: - When we apply tensile load to a body then it will plastically deformed then body will try to come in its original position. But it will not come because of its plasticity, then internal forces will set up in the body which will give strength and hardness to the body. This is known as work hardening. Also known as strain hardening.
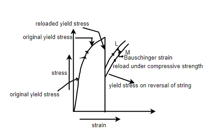
Bauschinger Effect: - It is related to the load reversed or cycle stress.
The effect was discovered by Johann Bauschinger. Under plastic deformation it would be normally observed that if crystalline materials same metals are loaded beyond elastic limit and load is removed and we applied the yield strength and proportional limit of the material would have increased. But if the stress is applied in the opposite direction then plastic deformation starts at lower yield stress than that of the initial deformation. This phenomenon is known as Bauschinger effect.
Recovery, recrystallization and grain growth: - If the temperature is raised sufficiently the metal attempts to approach equilibrium through the process name as
1) Recovery
2) Recrystallization
3) Green growth
Recovery: - Recovery is a low temperature phenomenon which result in the Restoration of the physical properties without any observable change in microstructure.
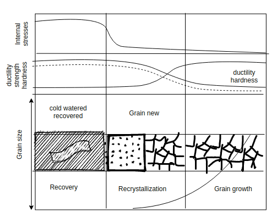
During recovery there is negligible effect on hardness whereas electrical resistance decreases rapidly towards the annealed value. The process of recovery is important for releasing internal stress in forging, molded and fabricated equipment’s without decreasing the strength acquired during cold water.
Recrystallization: - It is a process by which distorted grains of gold worked metal are replaced by new stream free grains during heating above the specific minimum temperature called recrystallization temperature.
Grain growth: - Grain growth is an increase in grain size when the material is held for longest X at temperature above recrystallization temperature or when it is heated to a higher temperature the grain size increases and there is decrease in hardness and strength and gain in ductility.
At a given temperature 't' the grain size D at a given time is given as
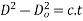
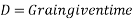
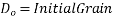
Fracture: - It is defined as the separation of an object or metal into two or more pieces under the action of stress. The fraction of a solid usually occurs due to the development of certain displacement discontinuities surfaces within the solid.
If displacement develop perpendicular to the surface of displacement it is called a normal tensile crack or simply a crack.
If displacement develop tangentially to the surface of displacement, it is called shear crack, slip band or dislocation.
Brittle fracture occurs with no prior deformation before fracture. Ductile fracture occurs when visible deformation does occur before separation.
Fracture strength or breaking strength is the stress when a specimen fails.
Types of fracture: -
1) Brittle fracture
2) Ductile fracture
Brittle fracture: - In brittle fracture, no apparent plastic deformation takes place before fracture. It involves little energy absorption and occurs at high speed up to 2133.6 m/s in steel.
Ductile fracture: - In ductile fracture extensive plastic deformation (necking) takes place before fracture. the term rupture ductile describes the ultimate failure of ductile material located in tension.
Creep: - It is a slope permanent deformation which occurs below the yield point. It occurs under steady loading conditions at a function of time.
In creep there are slips along crystallographic direction in individual crystal along with some grain deformation.
Fatigue: - Loading condition has to be cyclic or wearing over time. Fatigue life is determined from S-N curve based on the number of loading cycles.
Temperature: - Surface finish and heat treatment significantly affect the fatigue life.
Questions: -
Q1. Explain different types of cubic structure?
Q2. What are different types of defects in single atomic crystalline structure?
Q3. What do you mean by elastic and plastic deformation?
Q4. What is Bauschinger effect? Explain with diagram?
Q5. Define different types of material properties?
Q6. What is the difference in between brittle and ductile structure?
Q7. Explain creep and how does it occur?
Q8. Describe Recovery, Recrystallization and Grain growth?
Q9What is the difference in between resistivity and conductivity?
Q10.Define different types of mechanical properties of material?