UNIT 1
Fundamentals of Thermodynamics
Thermodynamics is a branch of science that deals with various forms of energy and its transformation from one form to another.
Thermodynamics deals with the behavior of gases when subjected to variations of pressure and temperature and the relation between heat and mechanical energy or work.
When a substance changes from one condition to another in a process, energy transformation may occur. Common processes are heating, cooling, expansion, compression.
Let us discuss basic definitions that we have already studied in Physics.
Working Substance or Medium
Thermodynamic process requires a carrier that would act as mode of transport of energy from or into the system. Such a medium is called as working substance or working medium.
For example, petrol is the working medium in SI engines whereas diesel is the working medium in CI engines.
System
Thermodynamic system is the region which is under observation for thermodynamic changes.
There are 3 types of systems thermodynamics
- Isolated systems,
- Closed system, and
- Open system
An isolated system cannot exchange either mass or energy with the surroundings.
A closed system exchanges energy with the surrounding but mass transfer is not possible.
An open system can exchange both mass and energy with the surroundings.
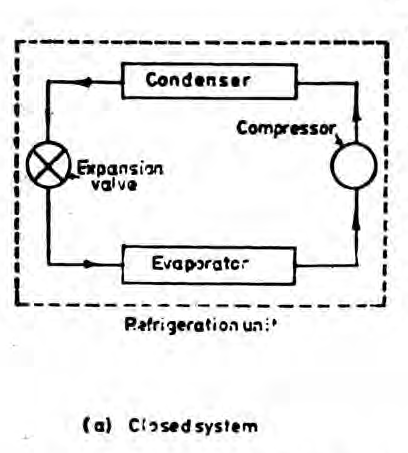
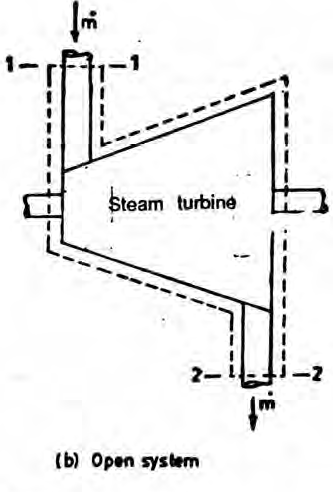
Basic Properties
Pressure, volume and temperature are named as basic properties or parameters as they may be determined by direct observations or simple measurements.
Pressure:
Pressure is a force applied per unit area. In SI (international system) units, the unit for pressure is the force of one Newton (N) acting on a square meter area, which is called the Pascal (Pa),
i.e. 1 Pa = 1 N/m2.
1 kilopascal = 1,000 pascals = 1 kPa
1 megapascal = 10,00,000 Pascals = 1 MPa
1 bar = 105 Pa = 100 kPa (kilopascal) = 0.1 MPa (megapascal),
and 1 standard atmosphere (atm) = 1,01,325 Pa
Volume:
Volume is defined as the space which the matter occupies and it is measured in cubic meters.
A widely used unit of volume is the liter which is given as
1 liter = 10-3 m3
The specific volume of a substance is its volume per unit mass. It is generally in cubic meters per kilogram.
1 kg of air at 0°C and under a pressure of 101.325 kPa (760 mm of Hg) has a volume of 0.7734 cubic meter.
Therefore, the specific volume of air under atmospheric conditions is 0.7734 m3/kg.
The density of a substance is mass per unit volume is generally stated in kilogram per cubic meter.
Density of air under atmospheric conditions is 1.293 kg/m3. Density is denoted by the symbol ρ.
Temperature:
The temperature of a substance is defined as measure of hotness or degree of coldness of a body. We know that energy flows from higher level to lower level in the universe. So, heat flows from higher temperature body to lower temperature body. It is normally measured in Kelvin in SI units.
Energy:
Energy may be defined as the capacity of a body to perform work. It is of many forms and can be converted from one form to another. Energy is a path function, that is it depends on the path followed by the process and not on the end states.
Work:
Work is a transient form of energy. Work is done when the application of some form of force results in change in state of the body. The unit of work is newton-metre (N-m), which is the product of a unit force (one newton) and unit distance (one metre) moved in the direction the force.
This unit of work is also known as joule (J),
that is 1 joule = 1 newton-metre (N-m)
In thermodynamics, work done by a system, is taken to be positive, and when work is done on a system it is taken to be negative.
Heat:
Heat is a form of energy which is transferred from one body to another due to temperature difference.
Internal Energy:
Energy possessed by body on account of kinetic energy of its infinite number of molecules is called as internal energy.
Δu = u2 – u1
Enthalpy:
Enthalpy is an energy term and is defined as follows:
H = u + pv
where u is the internal energy, p is the absolute pressure, and v is the volume.
ΔH = H2 - H1 = (u2 - u1) + p2v2 - p1v1
Zeroth Law of Thermodynamics:
If Body 1 is in thermal equilibrium with Body 3 and Body 2 is in thermal equilibrium with Body 3, it implies that Body 1 is also in thermal equilibrium with Body 2.
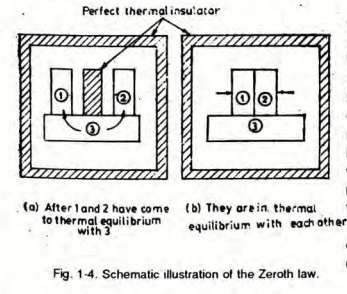
Thermal equilibrium is a state where no heat is transferred between two bodies in contact or between system and surrounding.
In thermodynamics, there are two ways to approach the theory:
In this system of analysis, the molecular level changes are not considered. It is rather concerned with behaviour of the system at a larger level.
This system is mathematically easier to analyze.
Only a few properties of the system are enough to define the state of the system.
The values of various properties are actually taken as average of the entire system, rather than calculating it for every molecule. For example, the value of temperature of a gas in cylinder is taken as average temperature of the cylinder space instead of expressing temperature of each and every molecule of gas.
The changes of properties considered in this analysis can be felt by the user.
This is also called as Classical Thermodynamics.
2. Microscopic Approach:
In this system of analysis, as the name suggests, the molecular level changes are considered. As the molecules of the system move randomly with different velocities and energies, the energy level of each molecule keeps on changing.
This system is much complex to analyze and requires quite advanced mathematical models to predict values of properties accurately.
The values of various properties are calculated for each molecule in the system. For example, velocity of each particle present in the system is analyzed and the process is quite cumbersome.
The changes occurring at molecular level cannot be felt by the user.
This is also called as Statistical Thermodynamics.
In order to define the thermodynamic system under state of equilibrium, some finite properties need to be known.
The statement that describes the values of some of these finite values of thermodynamic system is called as State postulate.
If the state postulate is given, say, it describes pressure and temperature of the system at equilibrium, by using various laws and charts, the values of remaining properties like volume, enthalpy, entropy can be found.
Hence, state postulate is important.
The exact condition of a substance is called its state.
Variables which determine the state of a substance are called its properties or parameters.
Pressure, temperature, volume, internal energy, enthalpy, entropy, heat are the properties that can be used to pinpoint the state of any thermodynamic system.
Minimum two of these are required to pinpoint the state of any substance.
When a thermodynamic system undergoes a change, the properties of the system change in accordance.
If we consider and monitor any two of these properties, say, pressure and volume, and the infinitesimal changes in these properties are plotted on a graph to know the exact set of values attained by these properties while the system undergoes change from state 1 to state 2, the locus of points is defined as Path followed by the system.
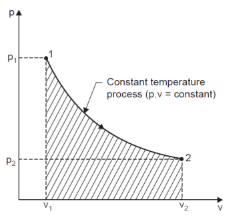
The path followed by some property of substance when it undergoes changes, it is said to have undergone a process.
Process is named according to its specification, i.e., constant pressure process, constant volume process, etc.
A cycle is a combination of processes so that the initial and final states of the system are the same. A thermodynamic cycle is also known as a cyclic operation of processes.
The properties that are depend only on the state of the system and do not vary according to the path followed by the system to reach a particular state are called as point functions.
These can be mathematically expressed by exact differentials.
Examples are pressure, temperature, volume, etc.
Exact differentials are the derivatives of some other functions as the name suggests.
These are used to express change in point functions. Examples are pressure change dP, volume change dV, etc.
The properties that depend on the path followed by system and not on the state of the system are called path functions. Examples are work, heat.
Inexact differentials are useful for expressing very minute quantities in these path functions.
The process that takes place at an infinitely slow pace, such that enough time is available to keep the system in homogeneous state, is called as quasi-static process.
All the processes in thermodynamic studies are considered to be quasi-static.
Various types of quasi-static processes considered are constant volume process, constant pressure process, constant temperature process.
In all these processes, it is assumed that the changes in the system take place at such a pace that the entire system gets enough time to change the value of various properties and remain homogeneous in nature.
It is the state of thermodynamic system, where all the properties of the system are same as the surroundings and there is no change occurring.
The system remains homogeneous in this state.
For example, when we say a body is in thermal equilibrium with the surroundings, it means that the temperature of every particle within the body is same as that of the surroundings and is not undergoing any thermal changes. There is no heat flow.
The temperature of a substance is defined as measure of hotness or degree of coldness of a body. We know that energy flows from higher level to lower level in the universe. So, heat flows from higher temperature body to lower temperature body.
Temperature is not a measure of quantity of energy possessed by the body but rather, it indicates level of internal energy possessed by the body
The absolute temperature is the temperature measured above the point of absolute zero. Absolute temperatures are expressed by the capital letter T.
Temperature Scales:
There are mainly four types of temperature scales.
- Freezing point of water: 32° F
- Boiling point of water: 212° F
- Coldest possible temperature: -459.67° F (absolute zero)
- Freezing point of water: 0° C
- Boiling point of water: 100° C
- Coldest possible temperature: -273.15° C (absolute zero)
- Fahrenheit to Celsius formula Celsius = (Fahrenheit - 32) / 1.8
- Freezing point of water: 273.15 K
- Boiling point of water: 373.15 K
- Coldest possible temperature: 0 K (absolute zero)
- You can convert from Celsius to Kelvin by adding 273.15 to Celsius temperature.
- Freezing point of water: 491.67 R
- Boiling point of water: 671.67 R
- Coldest possible temperature: 0 R (absolute zero)
- You can convert from Fahrenheit to Rankine by adding 459.67 to Fahrenheit temperature.
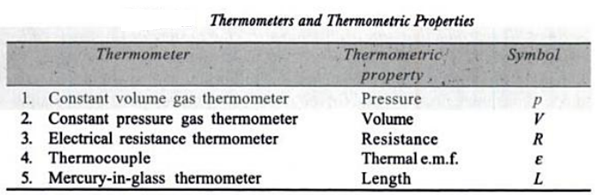
A thermometer is a device that is used to measure the temperature of a system.
Thermometers are based on the principle that some physical property of a system changes as the system’s temperature changes.
Mercury bulb thermometer is the most commonly used, since mercury has a very high co-efficient of thermal expansion.
Various types of thermometers that are used are tabulated below with their principle of operation.
International fixed points
Currently, ITS-90 scale is the standard temperature measurement scale in use.
It covers and defines 16 fixed points. It includes freezing, melting and triple points for substances like Water, gold, gallium, oxygen and so on.
These give a range of temperatures where the thermometers can be calibrated.
Example: Freezing point of water is fixed at 273.15 K or 0 ℃. Any thermometer can be calibrated for these values at freezing point of water.
The most important point in ITS-90 scale is the triple point of water which is fixed at 0.01 ℃.
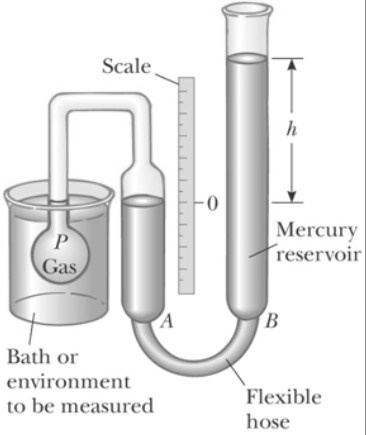
Constant Volume Gas Thermometer
These thermometers work on the principle that the temperature of any gas is directly proportional to the pressure of the gas at constant volume.
T = x + yP
This is the relation of temperature and pressure. They are linearly proportional to each other.
Once the constants x and y are known, the temperature can be found by putting the rigid vessel of thermometer in the medium and noting the gas pressure at thermal equilibrium state.
The thermometer can be calibrated by noting the changes in pressure at two distinct points like freezing and boiling point of water.
As only one straight lie can pass through any two points, the constants x and y can be found.
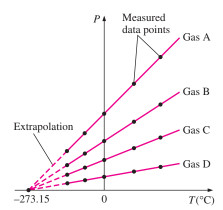
As the value of pressure and constants is now known, the value of temperature can be easily determined.
The values of constants x and y change as the medium changes.
All the lines plotted for different media, on extrapolation, intercept the X-axis at absolute zero temperature, that is –273.15 ℃.
Constant Pressure Gas Thermometer
These thermometers work on the principle that the temperature of any gas is directly proportional to the volume occupied by the gas at constant pressure.
T = x + yV
This is the relation of temperature and volume. They are linearly proportional to each other.
Once the constants x and y are known, the temperature can be found by putting the rigid vessel of thermometer in the medium and noting the volume at thermal equilibrium state.
As the temperature changes, the volume of the gas also changes. This change is noted and converted into equivalent temperature change by knowing the values of constants x and y.
The scale of the volume change is calibrated at two distinct known temperatures like freezing and boiling point of water.
Mercury In Glass Thermometer
This thermometer makes use of the principle that the metal changes its volume with change in temperature.
This is a constant pressure type thermometer.
Mercury is a metal that is very sensitive thermal changes.
Even slightest change in temperature results in visible change in volume of the liquid metal.
This is because the coefficient for thermal expansion of mercury is very high.
Volume at any temperature is given by
V = V0 + ɣT
Where, V = volume at temperature T2,
V0 = volume at temperature T1,
Ɣ = coefficient of thermal expansion for mercury
T = T2 – T1
The scale is calibrated by noting the volume of mercury at freezing point of water and boiling point of water.
It is the most widely used type of thermometer for normal usage.
Concept of heat and work
Heat and work are totally interconvertible in thermodynamics.
The relation between heat and work is given by the First law of thermodynamics.
Heat is the energy transfer occurring between two points due to difference in temperature.
Work is interaction between a system and its surroundings in the form of energy transfer.
Both heat and work are directional quantities.
Sign convention:
Heat absorbed by the system and work done by a system are taken positive.
Heat rejected by the system and work done on a system are negative.
First Law of Thermodynamics:
In simplest way, the First Law of Thermodynamics can be expressed as:
The total energy of the system increases if heat energy is added to the system and decreases if the energy is lost in the form of work produced by the system.
dE = dQ – dW
dQ = dE + dW
Mathematically, it is written as
∮ dW= ∮ dQ
It shows that total heat energy of the system is equal to the capacity of the system to produce work.
For a non-cyclic process, a closed system (In absence of KE and PE) executes, the work transferred and heat transferred may not be equal, and the difference between the two is accounted for by a change in internal energy, u of the system. This can be stated mathematically as
Q - W = Δu
Q = Δu + W
The main aim of Joules Experiment was to determine the relationship between the work done and the quantity of heat produced in mechanical system.
Construction and Working:
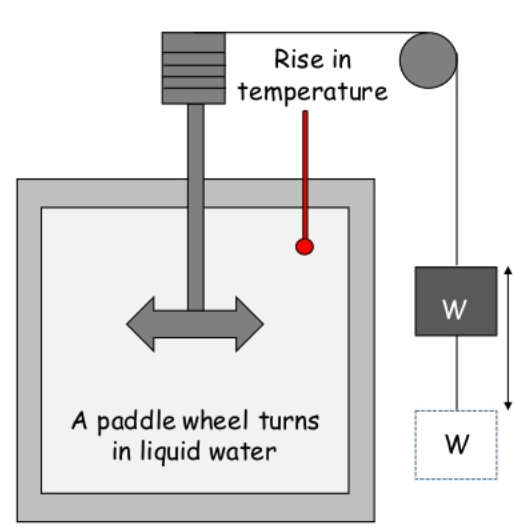
It consists of an insulated vessel. This vessel contains water.
A paddle wheel is submerged in water inside the vessel.
The paddle wheel can be turned by movement of weight attached to the string connecting the wheel.
As weight falls at constant speed, the paddle wheel is turned inside the water.
This produces work and results in increase in temperature of water which can be measured by thermometer.
This shows that work transferred to the system is converted into heat energy.
Hence, first law of thermodynamics is verified.
This shows the equivalence between heat and work.
The first law equation is
Q – W = △U
For a gas that approximates an ideal gas, the internal energy depends only on the temperature.
So, ΔU = 0 for constant temperature process.
Q = W
Using the ideal-gas equation
PV = mRT,
the work for a quasi-static isothermal process can be found to be
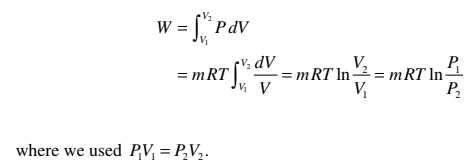
2. THE CONSTANT VOLUME PROCESS
The work for a constant volume quasistatic process is zero, since dV is zero.
For such a process the first law becomes
Q = ΔU
3. THE CONSTANT PRESSURE PROCESS
The first law, for a constant pressure quasistatic process,
Q = ΔH
4. THE ADIABATIC PROCESS
There are numerous examples of processes for which there is no, or negligibly
small, heat transfer. Such processes are called adiabatic processes.
Since, Q = 0, first law becomes
− δW = du
du + Pdv = 0
Cv dT + (RT/v) dv = 0
Integrating, (Cv / R) ln (T2 / T1) = - ln (v2 / v1)
Or
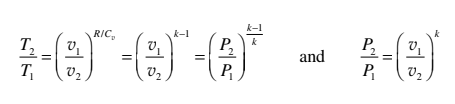
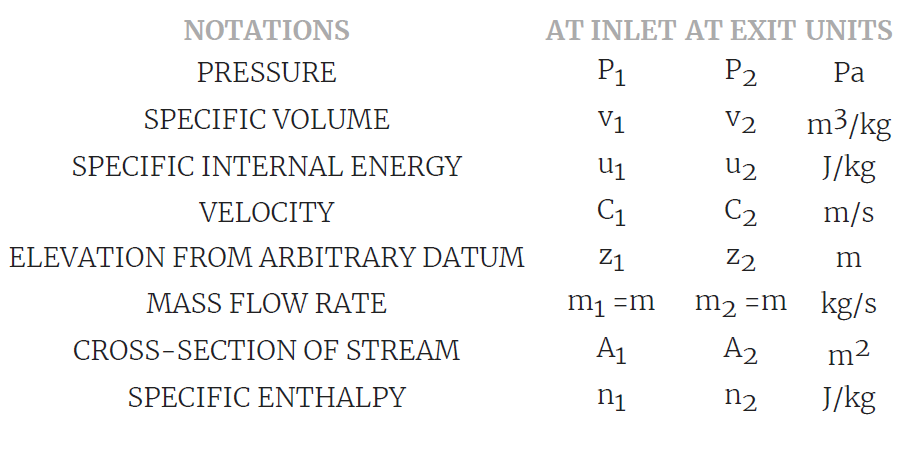
It is the system in which the mass flow rate entering the system is equal to mass flow rate exiting the system.
Mass flow rate in m1 = Mass flow rate out, m2 = constant = m
Consider the flow of fluid through a pipe of cross-sectional area A,
specific volume V, at Velocity C.
volume flow rate, vf = A (m2) × C (m/s)
Mass flow rate mf (kg/s) = vf / V
m =A x C / V = ρ x A x C= Continuity Equation
Assumptions-
S.F.E.E. on unit time basis -
Total energy flow rate into the control = total energy flow rate out of control volume.
Q+ m [Internal energy + Flow work + KE + PE ]1 = W +m [Internal energy + Flow work + KE + PE]2
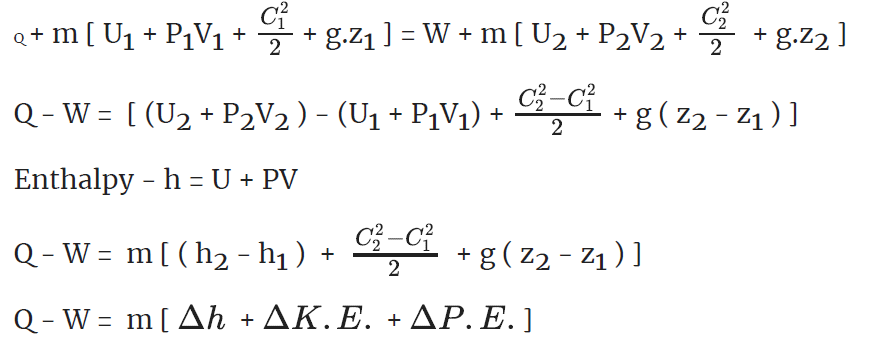
Boiler - A boiler is a device used for steam generation at constant pressure.
Heat is supplied to boiler drum externally by combustion of fuel in presence of air. Products of combustion are called Flue gases.
For a boiler - W =0, ΔK.E.=0 and Δ P.E.=0
On using these conditions S.F.E.Equation,
Q = m (h2 - h1)
Condenser -
A condenser is a device used for steam condensation at constant pressure.
For a condenser - W =0, ΔK.E.=0 and Δ P.E.=0
On using these conditions S.F.E.Equation,
Q = m (h1 – h2)
Nozzle: In case of a nozzle as the enthalpy of the fluid decreases and pressure drops simultaneously the flow of fluid is accelerated. This is generally used to convert the part of the energy of steam into kinetic energy of steam supplied to the turbine.
For this system, ∆PE=0,W=0,Q=0
Applying the energy equation to the system,
h1+(C12/2)=h2+(C22/2)h1+(C12/2)=h2+(C22/2)
Turbine: In a steam or gas turbine steam or gas is passed through the turbine and part of its energy is converted into work in the turbine. This output of the turbine runs a generator to produce electricity. The steam or gas leaves the turbine at lower pressure or temperature.
Applying energy equation to the system,
Here, Z1=Z2
h1+(C12/2)−Q=h2+(C22/2)+W
It is an imaginary concept. This would violate the first law of thermodynamics. It produces energy without any input and as we know that according to the law of energy conservation, energy cannot be created or destroyed but can only be converted from one form of energy to other form of energy.
Therefore, a machine that does not follow the first law of thermodynamics or law of conservation of energy is termed as perpetual motion machine of first kind i.e. PMM1