UNIT 5
Fuels and Combustion
Fuel is a substance that produces high amount of energy on controlled combustion. It may do so by reacting with some other substance. The reaction is highly exothermic in nature.
- Primary or natural fuels: These occur naturally in the nature. e.g. Coal, Wood, Crude Oil.
- Secondary or derived fuels: These are derived from the naturally occurring fuels. E.g. Petroleum products, charcoal.
- Liquid fuels: These are found as liquids in nature. E.g. Crude oil, Natural gasoline.
- Solid fuels: These are found as solids in nature. E.g. Wood and Coal.
- Gaseous fuels: These are found as gases in nature. E.g. Natural gas.
Proximate analysis:
This is an approximate analysis of fuel to find the basic composition of fuel. The results obtained are only approximate and proper values can be obtained by ultimate analysis.
Following are the important deductions of proximate analysis:
Ultimate analysis:
This is an analysis of fuel which gives exact composition of fuel. Percentage of different components can be found by completely combusting the fuel in the furnace.
Following are the important deductions of ultimate analysis:
Combustion is a process in which the fuel is oxidized producing enormous amounts of energy to be released. This energy is utilized in various processes like in IC engines or Thermal power plants.
During this process, constituents of fuel like C, S, H and N combine with oxygen to form their respective oxides.
The source of oxygen for this process is normally atmospheric air.
Atmospheric air consists of 21% Oxygen v/v and 23.2% w/v.
The quantity of air required to produce complete combustion of fuel is called as Stoichiometric Air.
Value of Stoichiometric air for different fuels varies.
Coal is mainly Carbon. Its complete combustion in presence of sufficient air is given by following reaction:
C + O2 = CO2 + 33.94 kJ/gm of C
12 gm of C + 2 x 16 gm of O2 = 44 gm of CO2 + heat
∴ 1 gm of C + 2.67 gm of O2 = 3.67 gm of CO2 + 33.94 kJ
The O2 content of air mass-wise is 23.2%.
∴ to produce 2.67 gm of O2 required in the above reaction, air required is,
100/23.2 x 2.67 = 11.5 gm
As per combustion theory assumptions, after combustion, 1 gm of C produces 3.67 gm of CO2 and 8.83 gm of N2.
Incomplete combustion reaction of coal:
2C + O2 = 2CO + 10.12 kJ/gm
(2 x 12) C + (2 x 16) O2 = (2 x 28) CO + heat
∴ 1 gm of C + 1.33 gm of O2 = 2.33 gm of CO + 10.12 kJ
∴ to produce 1.33 gm of O2 required in the above reaction, air required is,
100/23.2 x 1.33 = 5.75 gm
As per combustion theory assumptions, after combustion, 1 gm of C produces 2.33 gm of CO and 4.42 gm of N2.
S + O2 = SO2 + 9.141 kJ/gm
32 gm of S + 2 x 16 gm of O2 = 64 gm of SO2 + heat
∴ 1 gm of S + 1 gm of O2 = 2 gm of SO2 + 9.141 kJ
So, air required for complete combustion of each gm of Sulphur is
100/23.2 x 1 = 4.31 gm.
So complete combustion of each gm of Sulphur produces 2 gm of SO2 and 3.31 gm of N2.
2H2 + O2 = 2H2O + 142.67 kJ/gm
4 gm of H2 + 2 x 16 gm of O2 = 36 gm of H2O + heat
∴ 1 gm of H2 + 8 gm of O2 = 9 gm of H2O + 142.67 kJ
By applying combustion theory to combustion reactions of C, S and H2, it is found that 2.67 gm O2 is required for 1 gm C combustion, which implies 2.67 C gm O2 is required for C gm carbon, 1 gm O2 is required for 1 gm S combustion, which implies S gm O2 is required for S gm Sulphur and 8 gm O2 is required for 1 gm H combustion, which implies 8H gm O2 is required for H gm hydrogen.
Hence 1 gm of coal (fuel) which contains C gm carbon, S gm sulfur and H gm hydrogen, requires (2.67 C + S + 8 H) gm of O2 for complete combustion.
Some amount of O2 may be contained in the fuel itself in form of different compounds and it assists combustion process. If O is the original weight of the oxygen presents in 1 gm of fuel, net requirement of oxygen for sufficient coal combustion is (2.67 C + S + 8 H – O) gm.
Then, the amount of air required is
100/23.2 x (2.67 C + S + 8 H – O)
The ratio of air to fuel in AF mixture that would just completely combust the fuel without any excess air, is called as Equivalence ratio.
It is very important to obtain the quality and quantity of work produced by the fuel.
If, in AF mixture, A:F ratio is less than the Equivalence ratio, then the mixture is rich in fuel and there is no possibility of complete combustion.
If, in AF mixture, A:F ratio is more than the Equivalence ratio, then the mixture is lean in fuel and excess air is present which is unnecessary and causes heat losses in the process of combustion.
Calorific value: The amount of energy produced by complete combustion of fuel is called as Calorific value of that particular fuel.
It is normally expressed in kJ/kg.
Higher Calorific Value (HCV)
Fuel consists of elements that produce energy on combustion.
But the total amount of heat produced by combustion of fuel is not completely available to perform external work.
Some of the heat is utilized to evaporate the moisture content present in the fuel itself.
HCV is the maximum amount of energy that is produced on complete combustion of fuel. It is also generally termed as gross calorific value or GCV.
Lower Calorific Value (LCV)
If the amount of heat or energy utilized in evaporation of moisture content is removed from the HCV, we get LCV for the fuel. It is generally termed as net calorific value or NCV.
Calorific value of fuel can be determined by Bomb calorimeter experiment.
Calorimetry is the science of measuring quantities of heat, as distinct from “temperature”. The instruments used for such measurements are known as calorimeters.
Let's look at the Bomb Calorimeter in detail.
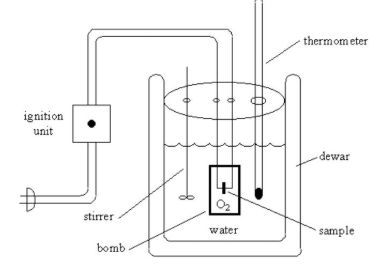
Four essential parts of bomb calorimeter:
Selection of Calorimeter:
The same calorimeters are used for all oxygen- combustible samples; solids and liquids alike.
Specially constructed, extra high strength bombs are available for burning explosives and similar hazardous materials, and bombs with a platinum lining or made of special corrosion resistant materials are available for use with samples which liberate unusual amounts of fluorine, chlorine or other corrosive combustion products.
Adiabatic calorimeters were the preferred choice in most industrial laboratories. Modern adiabatic jackets with microprocessor controls can approach the theoretical goal of eliminating all heat leaks, providing excellent precision in calorimetric tests, but specialized jackets and control systems are required.
Operation of Calorimeter:
Before a material with an unknown heat of combustion can be tested in a bomb calorimeter, heat capacity of the calorimeter must first be determined.
This value represents the sum of the heat capacities of the components in the calorimeter, notably the metal bomb, the bucket and the water in the bucket.
Since the exact amount of each of the metals used in the bomb and bucket is difficult to determine and continually changing with use, energy equivalents are determined empirically at regular intervals by burning a sample of a standard material with a known heat of combustion under controlled and reproducible operating conditions.
Benzoic acid is used almost exclusively as a reference material for fuel calorimetry because it burns completely in oxygen; it is not hygroscopic and is readily available in very pure form.
For example: Consider a standardization test in which 1.651 grams of standard benzoic acid (heat of combustion 6318 cal/g) produced a temperature rise of 3.047°C.
The energy equivalent (W) of the calorimeter is then calculated as follows:
W = (1.651) (6318)/3.047 =2416 cal/°C
It is important to note that the energy equivalent for any calorimeter depends on a set of operating conditions, and these conditions must be reproduced when the fuel sample is tested if the energy equivalent is to remain valid.
Fuel Test
After the energy equivalent has been determined, the calorimeter is ready for testing fuel samples. Samples of known weight are burned and the resultant temperature rise is measured and recorded.
The amount of heat obtained from each sample is then determined by multiplying the observed temperature rise by the energy equivalent of the calorimeter.
Then, by dividing this value by the weight of the sample we obtain the calorific value (heat of combustion) of the sample on a unit weight basis.
Assume a fuel sample weighing 0.9936 gram produced a temperature rise of 3.234°C in calorimeter with an energy equivalent of 2416 cal/°C.
The gross heat of combustion (Hg) is then determined by multiplying the temperature rise by the energy equivalent, and dividing this product by the weight of the sample, viz:
Hg = 7863 calories / gram.
For simplicity, we have omitted corrections for acids and fuse.
Calorimetric Corrections
The burning fuse wire in the bomb contributes additional heat to the bomb combustion. Since the amount of fuse wire consumed in each test may vary, the energy contributed by the fuse must be determined for each test and a correction applied to compensate for this variance.
Similarly, if any spiking material or combustion aid is used to promote complete combustion of a sample that is difficult to burn, the heat produced by the combustion aid must be subtracted from the total energy release.
Since combustion in the bomb takes place in an atmosphere of nearly pure oxygen at high temperature and pressure, several reactions occur which would not develop when burning the same material under normal atmospheric conditions. In normal combustion all sulfur in a fuel is oxidized to sulfur dioxide and discharged with the stack gases. But when the same material is burned in an oxygen bomb, the oxidation is carried further to trioxide which then reacts with moisture in the bomb to form sulfuric acid.
Likewise, in normal combustion nitrogen in the air is not affected. But when a fuel sample is burned in an oxygen bomb, some of the molecular nitrogen trapped in the bomb is oxidized and combined with water vapor to form nitric acid.
ASTM (American Society for Testing and Materials) and ISO (International Organization for Standardization) test methods contain procedures for calculating the correction which must be applied to account for the heat liberated in the formation of these acids.
Gross and Net Heat of Combustion
The results obtained from tests in a bomb calorimeter represent the gross heat of combustion of a sample, which includes the heat of vaporization given up when the newly formed water vapour produced by oxidation of hydrogen is condensed and cooled to the temperature of the bomb.
In nearly all industrial applications this water vapor escapes as steam in the flue gases and is not available for useful work.
To compensate for this loss, we can calculate the net heat of combustion by subtracting the latent heat of vaporization from the gross value obtained from the calorimeter, but this requires a knowledge of the hydrogen content of the sample.
If the hydrogen content is known, the net heat of combustion can be calculated as follows: Hn = Hg – (0.09H X 587)
where, Hn = net heating value, cal/gm,
Hg = gross heating value, cal/gm
H = weight percent hydrogen in the sample.
It was designed to get accurate values of the HCV and LCV of fuel.
Mainly, it was designed for use with gaseous fuels only.
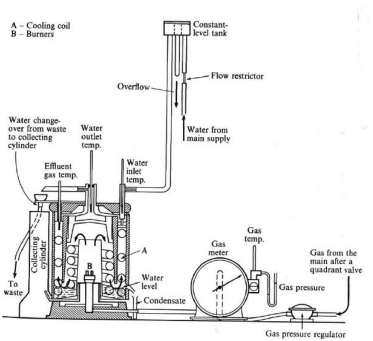
The gas calorimeter is designed to make sure that the heat from the burner flows up through the calorimeter container and back down again inside the container and back up again before exhausting.
From engineering point of view, we have to design methods that can extract higher heat or energy from the burning process and follow the process, with minimal losses of heat energy.
After some calculations and empirical procedures, with the state of the water in the combustion products, energy obtained from the combustion can be different.
The values that represent higher heating value and lower heating value are respectively when water is in liquid form and in gaseous form.
Basic theory is energy absorbed by the water when evaporating results in reduced heat output from the process.
So, with the help of this experiment, we can measure the difference of the HCV and LCV of the liquid petroleum gas. Higher calorific value has higher energy, as by the name of it than the LCV.
Some of the process is being disturbed by the higher heating values because of the condensed water, also on the other hand some of the process is being disturbed because of the formation of the water vapour.
In addition to this, these values are calculated under standard conditions because the ease of the comparison. So that the state that we are going to choose is much more important in practical cases.
Basic practical application is the use of fuel oil, gasoline or petrol, coke, coal, combustion water, foodstuffs and building materials. Also, these calorimeters can be used to measure the energy balance of Nano- material and ceramics.
Theory
We consider about the heating value, amount of energy released when a fuel is burnt completely is steady flow process and the products are returned to the state of the reactants.
There are two calorific values defined according to the state of the water at the combustion products.
1. Higher calorific value, higher heating value – when H2O in the product is in the liquid form
2. Lower calorific value, lower heating value – when H2O in the product is in the gaseous form.
HV is equal to the absolute value of the enthalpy of combustion of the fuel at a specified state.
HV = |hc|
Basic relation of HCV and LCV is HCV = LCV + (Nhfg)H2O
With the results of the practical, we can calculate the LCV.
• Gas volume = volume flow rate × time (cm3 )
• Gauge pressure = value × 10 × ( 1000 / 13600) (cm of Hg)
• Absolute pressure = Gauge pressure + Atmospheric pressure (cm of Hg)
• Correction of gas volume (V) : 𝑃1𝑉1 / 𝑇1 = 𝑃2𝑉2 / 𝑇2
• Increased temperature = 𝑇𝑒𝑚𝑝𝑒𝑟𝑎𝑡𝑢𝑟𝑒 𝑜𝑢𝑡 − 𝑇𝑒𝑚𝑝𝑒𝑟𝑎𝑡𝑢𝑟𝑒 𝑖𝑛 (𝐾)
• Latent heat (Q) = 𝑚𝑎𝑠𝑠 𝑜𝑓 𝑤𝑎𝑡𝑒𝑟 × 𝑠𝑝𝑒𝑐𝑡𝑓𝑖𝑐 𝑙𝑎𝑡𝑒𝑛𝑡 ℎ𝑒𝑎𝑡 (𝑘𝐽)
• HCV = (𝑀𝑎𝑠𝑠 𝑜𝑓 𝑐𝑜𝑜𝑙𝑖𝑛𝑔 𝑤𝑎𝑡𝑒𝑟×𝑠𝑝𝑒𝑐𝑖𝑓𝑖𝑐 ℎ𝑒𝑎𝑡 𝑐𝑎𝑝𝑎𝑐𝑖𝑡𝑦×𝑡𝑒𝑚𝑝𝑒𝑟𝑎𝑡𝑢𝑟𝑒 𝑖𝑛𝑐𝑟𝑒𝑚𝑒𝑛𝑡) / V𝑜𝑙𝑢𝑚𝑒 𝑜𝑓 𝑓𝑢𝑒𝑙 𝑢𝑠𝑒𝑑 𝑎𝑡 𝑔𝑖𝑣𝑒𝑛 𝑐𝑜𝑛𝑑𝑖𝑡𝑖𝑜n
Procedure
1. The upper part of the calorimeter is removed, open the gas flow and light up the burner.
2. Constant pressure head is set to the apparatus and open the water flow.
3. The stop watch is set and at that moment water direction is changed to collect the water that is circulating through the apparatus.
4. Water is collected that is circulating through the calorimeter for 5 minutes.
5. Temperature readings are taken after 5 minutes of collecting the water.
6. Volume flow rate is taken by the flow meter and readings are calculated with the help of given chart.
7. All the values are noted down and the HCV can be calculated.
In the proximate and ultimate analysis, the analysis of fuel was done to find out the combustion properties of the fuel.
In Orsat apparatus, we can analyze the exhaust from combustion by weight. This weight analysis can be then converted to volume analysis by using Avogadro’s hypothesis.
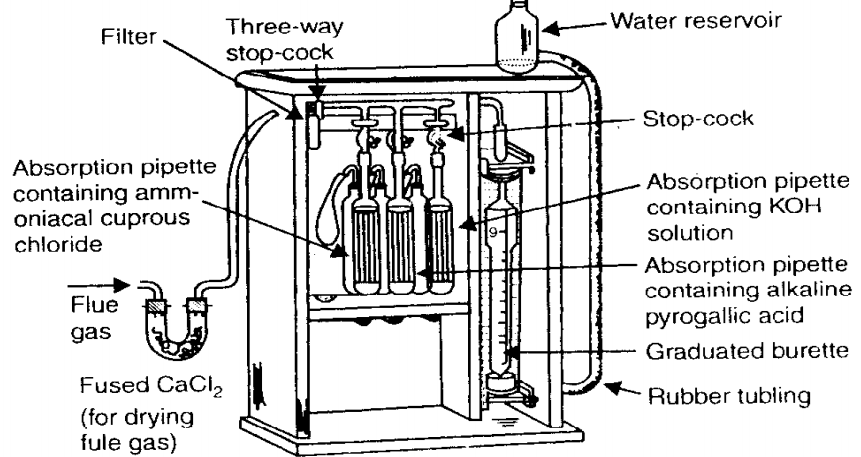
In order to know whether the combustion occurring is complete or incomplete, analysis of flue gases is done.
CH4 + 2O2 ------> CO2 + 2H2O
2CH4 + 3O2 ----------> 2CO + 4H2O
If combustion of fuel is complete then CO2 is released.
If combustion of fuel is incomplete then CO if released.
Thus, it can be affirmed that
CONSTRUCTION
• It is made of a water-jacketed measuring burette which is connected in series to 3 absorption bulbs, each through an individual stop-cock.
• The opposite side is provided with a 3-way stop-cock, one end of which is connected to a U-tube stuffed with glass wool (to avoid entry of smoke particles, etc.)
• The burette is surrounded by a jacket filled with water. This helps to keep the temperature of the gas constant.
• The bottom end of the burette is connected to reservoir of water through a long tube made of rubber.
• Each absorption bulb is filled with different solution to help in absorption of different components from the flue gases.
• The first bulb has 250 gm of KOH in half a litre distilled water which will absorb only CO2.
• The second bulb has 25 gm of pyrogallic acid mixed with 200 gm of KOH in half a litre distilled water. This will absorb CO2 and O2.
• The third bulb has 100 gm of cuprous chloride with 125 ml of liquified ammonia and 375 ml of water. This is capable of absorbing CO, CO2 and O2.
WORKING
• Flue gases are first passed through the hygroscopic Calcium chloride which would remove all the moisture present in the flue gases, if any.
• When the 3-way stop-cock is opened, the burette is filled with flue gases.
• This entire arrangement is surrounded by a jacket filled with water to maintain the temperature.
• The stop-cock for KOH is opened and the flue gases enter the KOH bulb.
• All the CO2 is absorbed here.
• Same procedure is repeated for the other two bulbs.
• The increase in volume of the bulb contents indicates the various components absorbed in each bulb.
Enthalpy of formation
It is also referred to as Heat of Formation.
It is the enthalpy change that occurs when it is formed from its constituents.
The enthalpy of change measured under standard conditions is called Standard Enthalpy of Formation.
The standard state is taken as pressure of 1 atm at 25 degrees C temperature.
When 1 mole of a substance is formed under these conditions, the corresponding value of enthalpy of formation is called as Standard Enthalpy of Formation.
Adiabatic Flame Temperature
In an adiabatic process (Q = 0), the temperature attained by the products of combustion is highest. This maximum possible temperature is called Adiabatic Flame Temperature.
This value depends on
For a particular fuel, at a particular state burned with air at a particular state, the adiabatic flame temperature attains its maximum value when complete combustion occurs with the theoretical amount of air. The actual temperature encountered inside the reaction chamber is always lower than the theoretical adiabatic flame temperature.