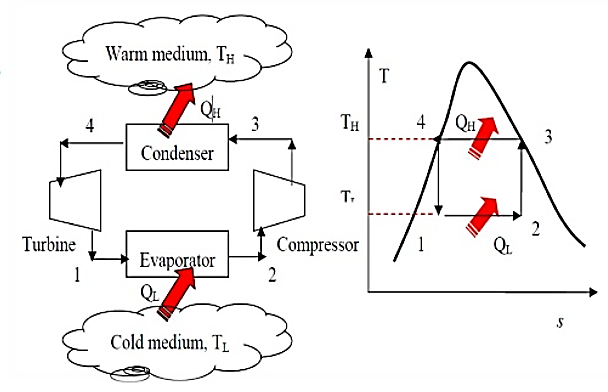
Fig. Schematic of a Carnot refrigerator and T-S diagram for the reversed Carnot cycle.
Reversed Carnot Cycle consists of 4 processes:
1. Adiabatic Compression
2. Isothermal Compression
3. Adiabatic Expansion
4. Isothermal Expansion
a) (1-2) Adiabatic compression of the working fluid with the aid of external work. The temperature of the fluid rises from T2 to T1.
b) (2-3) Isothermal compression of the working fluid during which heat is rejected at constant high temperature T1.
c) (3-4) Adiabatic expansion of the working fluid. The temperature of the working fluid falls from T2 to T1.
d) (4-1) Isothermal expansion of air where heat is absorbed at low temperature T2 from the space being cooled.
Its improved refrigeration cycle which uses fluid instead of air as working substance. These systems belong to the general class of vapor cycles, wherein the working refrigerant undergoes phase change at least during one process. The working substance is circulated in the system in which it alternatively evaporates and condenses; thus, it undergoes a phase change. Refrigeration is obtained as the refrigerant evaporates at low temperatures. The input to the system is in the form of mechanical energy required to run the compressor. Hence these systems are also called as mechanical refrigeration systems.
Assumptions for ideal vapor-compression cycle are:
Compression process is isentropic Main components are:
Refrigeration is the process by which heat (thermal energy) is transferred from a low temperature body to a high temperature body and the heat that is removed from the low temperature body accounts for the refrigeration effect.
Compressor power:
Coefficient of performance (COP):
COP = desired output / required input
COP = Cooling effect/ work input = QL/Wnet,input
A rule of thumb is that the COP improves by 2 to 4 percent for each °C the evaporating temperature is raised or the condensing temperature is lowered.
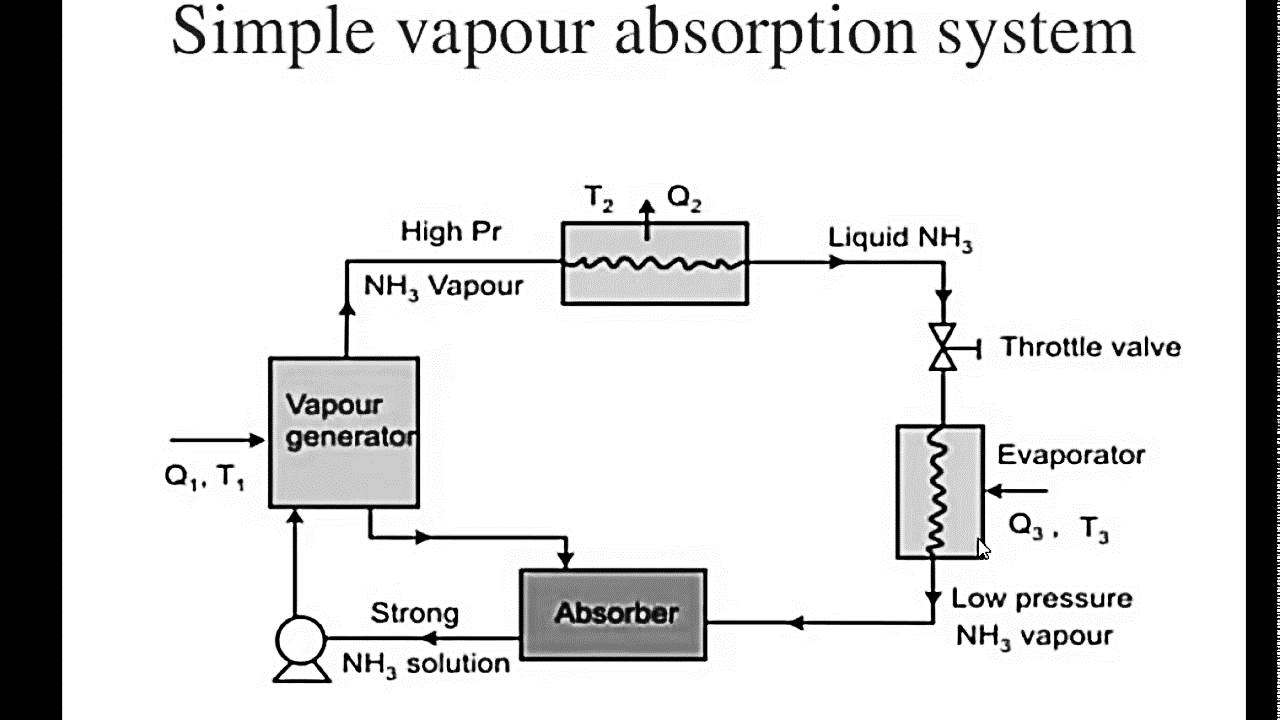
Fig. Simple Vapour Absorption Cycle
Vapour Compression Cycle (VCC) | Vapour AbsorptionCycle (VAC) | |
1 | Vapor compression has high C.O. P | It has low C.O. P |
2 | The charging of refrigerant is simple. | The charging of refrigerant is difficult. |
3 | A possibility of leakage of refrigerant is more. | A possibility of leakage of refrigerant is less. |
4 | Performance is adversely affected by part loads. | Reduced loads have no effect on its performance. |
5 | It cannot be located outside without shelter. | It can be located outside without shelter. |
6 | It is less bulky. | It is bulky. |
7 | Liquid traces in the suction line may damage the compressor. | Liquid traces in the refrigerant at the exit of the evaporator is not harmful to any component. |
8 | Wear and tear are high. | Wear and tear are less |
9 | It has a compressor and a motor. Therefore, It is more noise in operation. | It has a pump only a moving part. hence it is quiet in operation. |
10 | It uses high-grade work energy. It needs electrical energy for its operation. | It uses low-grade heat energy therefore, It can operate on exhaust from I.C engines or on Kerosene lamp or process heat. |
Psychrometry Or Psychrometry is the science dealing with the physical laws of air – water vapour mixtures.
When designing an air conditioning system, the temperature and moisture content of the air to be conditioned, and the same properties of the air needed to produce the desired air conditioning effect.
In other words, we can say that Psychrometry is the study of moist air or mixture of dry air and water vapour.
It is the study of the properties of mixtures of air and water vapour. Atmospheric air is a mixture of many gases plus water vapour and a number of pollutants. The amount of water vapour and pollutants vary from place to place. The concentration of water vapour and pollutants decrease with altitude. Above an altitude of about 10 km, atmospheric air consists of only dry air. The pollutants have to be filtered out before processing the air. Hence, what we process is essentially a mixture of various gases that constitute air and water vapour. This mixture is known as moist air.
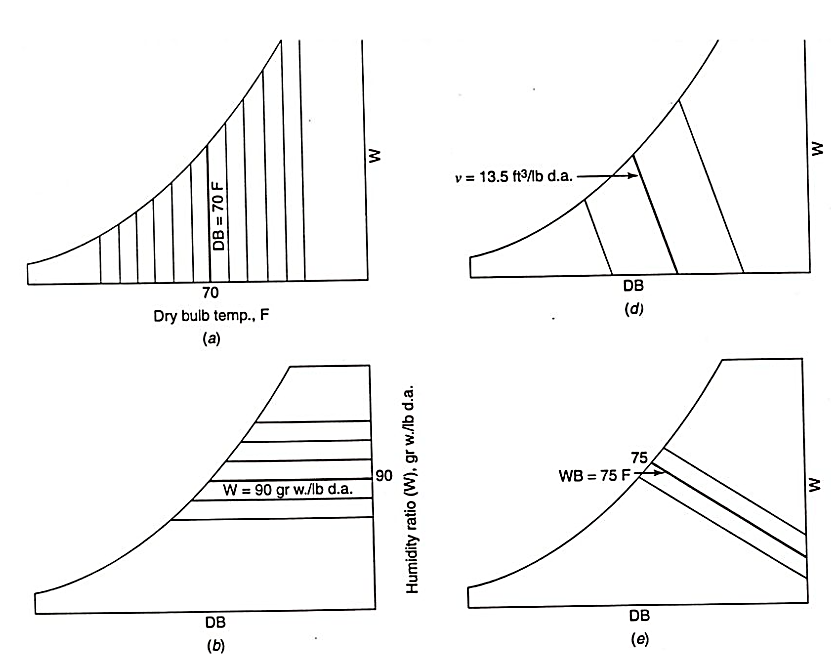
Psychrometric properties:
Dew point temperature is determined by moving from a state point horizontally to the left along lines of constant humidity ratio until the upper, curved, saturation temperature boundary is reached.
Actual weight of water in an air – water vapour mixture.
Amount of moisture per unit of dry air.
Can be defined as, W=m/G
The dry-bulb temperature is the temperature indicated by a thermometer exposed to the air in a place sheltered from direct solar radiation. The term dry-bulb is customarily added to temperature to distinguish it from wet-bulb and dew point temperature.
Wet bulb temperature is the temperature recorded by thermometer when the bulb is enveloped by cotton wick saturated with water.
The accuracy of a simple wet-bulb thermometer depends on how fast air passes over the bulb and how well the thermometer is shielded from the radiant temperature of its surroundings.
Amount of moisture that a given amount of air is holding = Amount of moisture that a given amount of air can hold 50% RH 100% RH - Saturated (percentage)
Specific humidity is defined as the proportion of the mass of water vapour per unit mass of the moist air sample (dry air plus the water vapour); it is closely related to humidity ratio and always lower in value.
The mass of water vapor per unit volume of air containing the water vapor. This quantity is also known as the water vapour density.
The psychrometric ratio is the ratio of the heat transfer coefficient to the product of mass transfer coefficient and humid heat at a wetted surface.
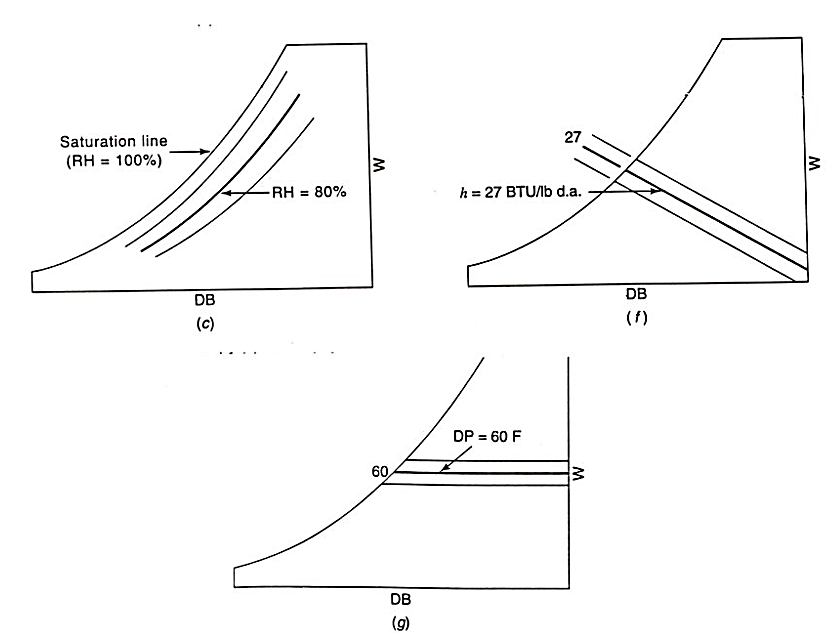
Dew Point Temperature: The temperature of moist air saturated at the same pressure and humidity ratio. Or more simply the temperature at which water vapor will begin to condense from a sample of air.
Dry-Bulb Temperature: The temperature of air read on a standard thermometer indicating its thermal state.
Enthalpy: The thermodynamic property defined as energy per unit mass of dry air commonly used to define the internal energy of moist air.
Humidity Ratio: The ratio of the mass of water vapor to the mass of dry air of a sample.
Relative Humidity: The ratio of mole fraction of water vapor in a given moist air sample to the mole fraction in a saturated air sample at the same temperature and pressure.
Specific Volume: The ratio of the total volume of air to the mass of dry air in a sample.
Wet-Bulb Temperature: The equilibrium temperature reached as water evaporates from a thoroughly wetted psychrometer wick into an airstream.
It is addition of heat to moist air without the addition of moisture. It follows a constant humidity ratio line on the psychrometric chart.
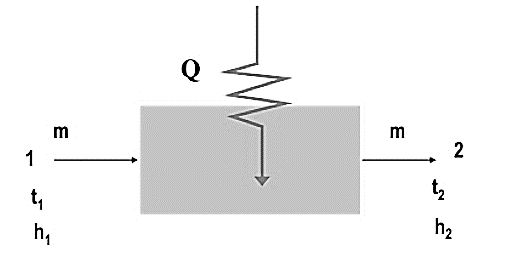
Fig. Sensible Heating
It is the removal of heat from moist air without the removal of moisture. It also follows a constant W on the psychrometric chart.
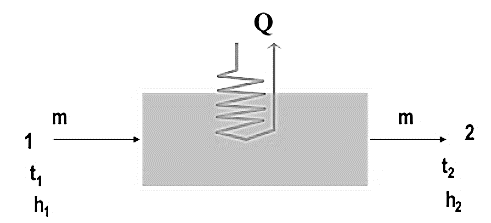
Fig. Sensible Cooling
In cooling coils temp of air reduces and the saturation point (dew point) is reached. O Further cooling results in reduction of absolute humidity.
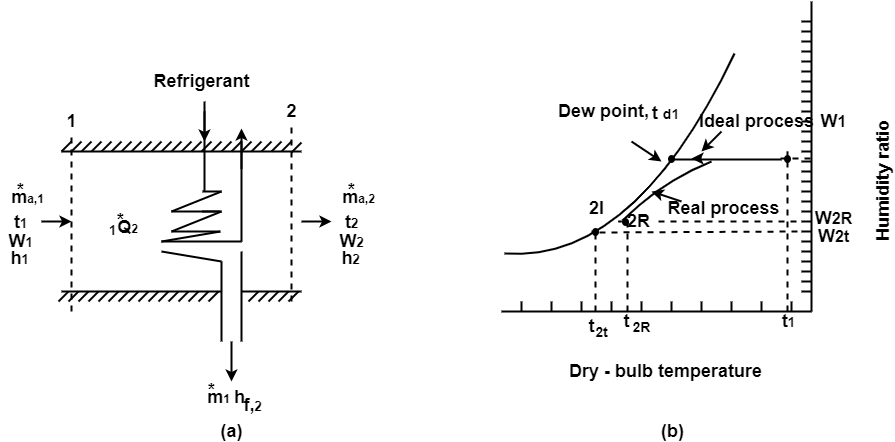
Fig. Dehumidification
It is the addition of moisture to moist air without the addition of heat.
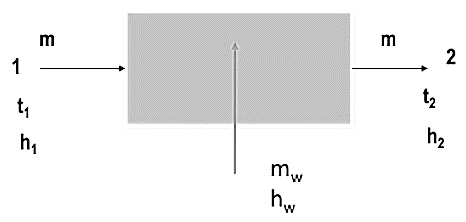
Fig. Humidifying
A psychrometric process which involves the cooling without heat loss or gain. Sensible heat lost by air is converted to latent heat in the added water vapor.
Fig. Adiabatic Cooling
A psychrometric process that involves no net heat loss or gain during the mixing of two air streams.
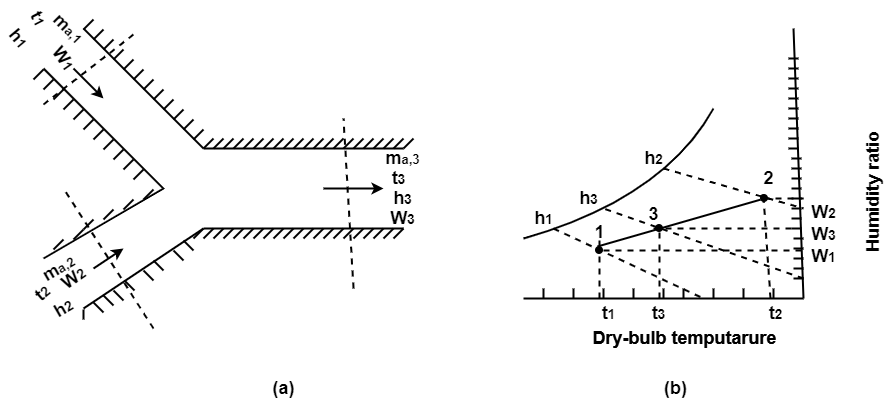
Fig. Adiabatic Mixing
a) Extensively used by engineers.
b) Chart displays DBT, WBT, W, RH, DPT, specific volume and enthalpy.
c) Each point on the chart represents one moist air condition.
d) Locating the point from known conditions will allow determining the other unknown conditions.
e) One chart for a specified atmospheric pressure.
f) Two additional independent states needed to fix state on chart.
g) X-axis is dry-bulb temperature; and
h) Y-axis is humidity ratio
Reference:
1) V. Ganesan: Internal Combustion Engines, Tata McGraw-Hill
2) M.L. Mathur and R.P. Sharma: A course in Internal combustion engines, Dhanpat Rai
3) H.N. Gupta, Fundamentals of Internal Combustion Engines, PHI Learning Pvt. Ltd.