UNIT - 1
INTRODUCTION
Thermodynamics state: State is the condition of system identified by thermodynamics properties. The state of a system in thermodynamics equilibrium can be represented by a point with properties as coordinates .the system is said to exist at a definite state when all the properties have a definite value.
Thermodynamics process represents a transition in which a system changes from one state to another. When the path is completely specified then the change of state is called a process. A Process is defined as the transformation of the system from one fixed state to another fixed state .When any one of the properties changes, the working substance or system is said to have undergone a process.
Some of the processes are identified by special names as given below:
- Isobaric process (constant pressure process.
- Isochoric process (constant volume process)
- Isothermal process (constant temperature process)
- Isentropic process (constant entropy process)
- Adiabatic process(perfectly insulated process)
Cycle: Any process or series of processes whose end state are identical is called a cycle. Or Series of thermodynamic processes, the end states of which are identical, is called a thermodynamic cycle. In a thermodynamic cycle, chemical composition of the working fluid during the process does not change. Thus all the properties in the initial and final states remain unchanged.
Example - water that circulates through the steam power plant. There is a change of phase during the process, but the end states are same.
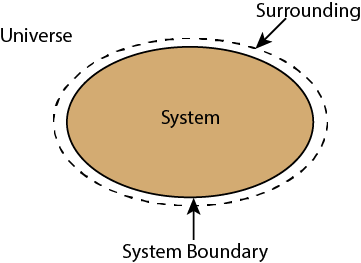
Whenever a change is to be analysed it is essential to specify the region in which the change take place. This is done by drawing a boundary around the region. Boundary may be real or imaginary.
Everything within the boundary is called the system. The region outside the boundary is called surroundings
There are three types of Thermodynamics systems:
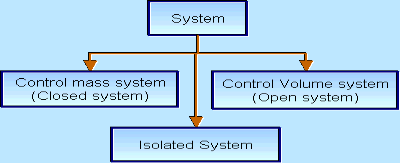
1 – Open system (control volume system): If mass and energy both cross the boundary of a system it is called open system .in open system both mass and energy can transfer across the boundaries and the mass within the system may not be constant .most of the engineering devices are open systems involving flow of fluids through them.
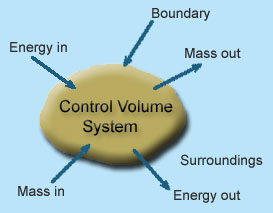
Example of open system
- Boiler – In boiler water convert from liquid to vapour phase. So mass and energy both transfer the boundary.
- Hydraulic turbine wheel – In this device potential energy of water convert into mechanical works. Water enters the wheel from head race side and leaves it to the tail race from the other end, and as such mass cross the system boundary.
- Motor-car engine – The engine sucks charge which is mixture of air and petrol and finally exhausts the gases to the surrounding atmosphere.
2 – Closed system (control mass system): If the mass within the boundary of the system does not change, it is called closed system. A closed system does not allow any mass transfer across the boundary but allow transfer of energy across the boundary.
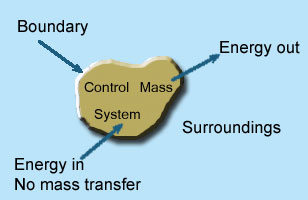
Example of closed system
- Piston cylinder assembly –the gas can reject or receive heat, expand or contract but no mass cross the boundary of system.
- Ignition system – electric energy cross the boundary to cause a spark between electrodes an initiate combustion .there is heat transfer but not mass across the system.
Pressure cooker, refrigerator, ice cream freezer etc. are the example of closed system.
3 - Isolated system : Neither mass nor energy cross the boundary of a system .it is called an isolated system . In this no mass and energy cross the system
Example of isolated system
- Universe can be considered as an isolated system
- Fluid enclosed in a perfectly insulated closed vessel (thermos flask)
Heat: Heat is that form of energy for which the driving force is temperature difference. It is also defines as the heat is energy transferred across the boundary of a system because of a temperature difference between the system and surrounding. Heat is transient quantity which exists only as it crosses the boundary of a system.
Heat flowing into a system is considered positive while heat flowing out of a system is considered negative.
Work: In thermodynamics, work performed by a system is the energy transferred by the system to its surroundings. Kinetic energy, potential energy and internal energy are forms of energy that are properties of a system. Work is a form of energy, but it is energy in transit. A system contains no work, work is a process done by or on a system. In general, work is defined for mechanical systems as the action of a force on an object through a distance.
Work done by a system on its surrounding is considered positive while work done on the system by the surroundings is considered negative .
We know that ,work = force x displacement
But in case of thermodynamics works we take force is the product of a pressure p at the boundary and the area A of the moving boundary therefore
DW = pAdl
Where dW is the work done during an infinite displacement dl of the boundary .
Since Adl = dv = change in volume
DW = pdv
The conditions under which the above expression is valid are
- The system is closed
- There are no viscous effects within the system.
- The pressure and other properties have the same value on all boundaries of the system
- The effects due to gravity electricity or magnetism are negligible
Internal energy
Internal energy is the sum of the kinetic energies and the potential energies of the molecules. We denote the internal energy of the system by U. Energy possessed by the atoms or molecules by virtue of their motion is kinetic energy. Since there is a force of attraction between two molecules, they possess some potential energy. The sum of total kinetic and potential energies of atoms or molecules constituting a system is the internal energy of the system.
The internal energy of a system depends upon the state of the system. It has a definite value for a definite thermodynamic state. It is not a measurable quantity. If a system starting from one state passes through different states and finally returns to its initial state, then the change in its internal energy will be zero.
Sometimes our body start to sweat and feel warm when we are in a room full of people and the sweating becomes excessive if the room size is small. This happens because our body is trying to cool off hence heat transfers from our body in the form of ‘sweat’. This entails the first law of thermodynamics.
The first law of thermodynamics states that the total energy of an isolated system is constant. Energy can be transformed from one form to another, but can neither be created nor destroyed.
According to this law, some of the heat given to system is used to change the internal energy while the rest in doing work by the system. Mathematically,
ΔQ=ΔU+ΔW
Where,
ΔQ = Heat supplied to the system
ΔW= Work done by the system.
ΔU = Change in the internal energy of the system.
If Q is positive, then there is a net heat transfer into the system, if W is positive, then there is work done by the system. So positive Q adds energy to the system and positive W takes energy from the system.
It can also be represented as ΔU=ΔQ−W
We can say that internal energy tends to increase when heat is given to the system and vice versa.
A steady flow process is one in which matter and energy flow steadily in and out of an open system. In a steady flow process, the properties of the flow remain unchanged with time, that is, the properties are frozen in time. But, the properties need not be the same in all points of the flow.
It is very common for a beginner to confuse the term steady with the term equilibrium. But, they are not the same. When a system is at a steady state, the properties at any point in the system are steady in time, but may vary from one point to another point. The temperature at the inlet, for example, may differ from that at the outlet. But, each temperature, whatever its value, remains constant in time in a steady flow process. When a system is at an equilibrium state, the properties are steady in time and uniform in space. By properties being uniform in space, we mean that a property, such as pressure, has the same value at each and every point in the system.
A steady flow is one that remains unchanged with time, and therefore a steady flow has the following characteristics:
- No property at any given location within the system boundary changes with time. That also means, during an entire steady flow process, the total volume Vs of the system remains a constant, the total mass ms. of the system remains a constant, and that the total energy content Es of the system remains a constant.
- Since the system remains unchanged with time during a steady flow process, the system boundary also remains the same
- No property at an inlet or at an exit to the open system changes with time. That means that during a steady flow process, the mass flow rate, the energy flow rate, pressure, temperature, specific (or molar) volume, specific (or molar) internal energy, specific (or molar)enthalpy, and the velocity of flow at an inlet or at an exit remain constant.
- Rates at which heat and work are transferred across the boundary of the system remain unchanged.
The application of the first law of thermodynamics to steady-flow processes is defined by two fundamental principles:
(a) Steady mass flow rate m = pAu (mass continuity)
Where p = fluid density
A = duct area
u = fluid velocity
And m = steady mass flow rate (dm/dt)·
(This is usually recast as mv = uA since v = 1/p.)
(b) Conservation of energy (the steady-flow energy equation or SFEE)
Q1-2 –W1-2 =m [(h 2 -h1) + ½ (u22 –u12) + g(Z2 -Zl)]
Where Z = height above datum .
Note that very often gz is negligible compared with other terms and the
Reduced SFEE is written as
Q2 -W2 =m [(h 2 -h1) + ½ (u22 –u12) ]
In per unit mass flow rate. Q/m=q , W/m= w
q2 - w2 = (h2 – h1) + ½ (u22 –u12)
An even further reduction by negligible kinetic energy terms here
q2 - w2 = (h2 – h1)
Many processes in reality approximate closely to steady flow, example steady conditions in a steam power plant after the start up transient is over and steady conditions exist at all points in the system.
Here two essential characteristics of steady flow:
(a) m is constant,
(b) The properties at any station are invariable with time .
The one obvious application of the SFEE where gz is prominent is in a hydroelectric plant where a change in z is essential for power production.
- The limitation of the first law of thermodynamics is that it does not say anything about the direction of flow of heat.
- It does not say anything whether the process is a spontaneous process or not.
- The reverse process is not possible. In actual practice, the heat doesn’t convert completely into work. If it would have been possible to convert the whole heat into work, then we could drive ships across the ocean by extracting heat from the water of the ocean.
Question 1- Find out the internal energy of a system which has constant volume and the heat around the system is increased by 100 J ?
Answer 1 - Given, q = 100 J
Since
The gas is in constant volume, Δv=0
w= p Δv=0
The equation for internal energy is,
ΔQ=ΔU+ΔW
ΔU= ΔQ – ΔW (ΔW = 0)
ΔU = ΔQ = 100 J
Question 2 - 3000 J of heat is added to a system and 2500 J of work is done by the system. What is the change in internal energy of the system?
Answer 2 – given in question
Heat (Q) = +3000 Joule
Work (W) = +2500 Joule
Find the change in internal energy of the system ?
We know that The equation of the first law of thermodynamics
ΔQ=ΔU+ΔW
Also written as
ΔU = ΔQ - ΔW
The sign conventions
Q is positive if the heat added to the system
W is positive if work is done by the system
Q is negative if heat leaves the system
W is negative if work is done on the system
The change in internal energy of the system :
ΔU = 3000-2500
ΔU = 500 Joule
Internal energy increases by 500 Joule.
The second law of thermodynamics can be understood by following two statements.
Kelvin-Planck statement
It is impossible to convert all the heat extracted from a hot body into work. In the heat engine, the working substance takes heat from the hot body, converts a part of it into work and gives the rest to the cold body. There is no engine that can convert all the heat taken from the source into work, without giving any heat into the sink. This means that for obtaining continuous work, a sink is necessary.
Clausius statement
It is not at all possible to transfer heat from a cold body to a hot body without the expenditure of work by an external energy source or its states that the heat energy cannot transfer from a body at a lower temperature to a body at a higher temperature without the addition of energy.
For example - There is a refrigerator that transfers an amount of heat from a cold body to a hot body without having any supply of external energy. So this is the violation of Clausius statement. Now suppose an engine working between the same hot and cold bodies takes in heat from hot body converts a part W into work and gives the remaining heat to the cold body.
The engine alone does not violate the second law of thermodynamics. But if the engine and refrigerator combine together, they form a device that takes up all the heat from the hot body and converts all into work without giving up any amount to the cold body. It violets the Kelvin-Planck statement. So we say that the two statements of the second law of thermodynamics are equal in all respects