UNIT – II
INSTRUMENTAL METHODS OF CHEMICAL ANALYSIS
Chemical analysis relies on the use of measurements. It is divided into two categories that depend on the manner in which the assays are performed. Classical analysis is also known as wet chemical analysis. It consists of analytical techniques that use no mechanical or electronic instruments other than a balance. The method usually relies on chemical reactions between the material being analyzed and a reagent that is added to the analyte. Wet techniques often depend on the formation of a product of the chemical reaction that is easily detected and measured. E.g.: product could be colored or could be a solid that precipitates from a solution.
Most of the chemical analysis is placed into the second category that is instrumental analysis. It involves the use of an instrument other than a balance to perform the analysis. A wide assortment of instrumentation is available to the analyst. In some cases, the instrument is used to characterize a chemical reaction between the analyte and an added reagent; in others, it is used to measure a property of the analyte.
Advantages of instrumental methods:-
- The minute sample quantity is required.
- The instrumental method determination is considerably fast.
- Complex mixture can be analysed either with or without their separation.
- Sufficient reliability and accuracy of results are obtained by instrumental method.
- When non-instrumental method is not possible, instrumental method is the only answer to the problem.
Limitation of instrumental methods:-
- Instrumental methods are costly because of cost, maintenance and trained personnel required for their handling.
- The used instrument defines sensitivity and accuracy.
- Specialized training for handling instrument is required.
- There is frequent need of checking results with other methods.
- In some cases, instrumental method may not be specific.
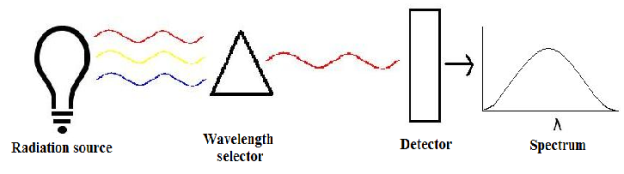
A spectrometer is used to measure wavelengths of electromagnetic radiation that has interacted with a sample. Incident light can be reflected off, absorbed by, or transmitted through a sample. The way the incident light changes during the interaction with the sample is characteristic of the sample. A spectrometer measures this change over a range of incident wavelengths.
|
There are three main components in all spectrometers; these components can vary widely between instruments for specific applications and levels of resolution. Generally, these components produce the electromagnetic radiation, somehow narrows the electromagnetic radiation to a specified range, and then detect the resulting electromagnetic radiation after is has interacted with the sample.
2.3.1 Introduction
UV – Visible spectroscopy
- Ultraviolet – visible spectroscopy
- Spectroscopy is the branch of science that deals with the study of interaction of electromagnetic radiations with matter.
- The spectroscopic techniques make use of either absorbed or emitted or scattered electromagnetic radiation by atoms or molecules present in the substance which help in qualitative and quantitative analysis of substances.
Electromagnetic radiations: -
- Electromagnetic radiations are a form of energy that is transmitted through space with high velocity.
- Electromagnetic radiations consist of discrete packets of energy which are called photons.
- A photon consists of an oscillating electric field (E) and an oscillating magnetic field (M)
Terms: -
- Wavelength (X): -
Wavelength of radiation is the distance between two crests or peaks of either electric or magnetic component. it is expressed in meters.
2. Frequency (v): -
It is the number of waves that pass through a fixed point in unit time. it is expressed in reciprocal second (s-1) or hertz (hz).
3. Wave number (v): -
It is reciprocal of wavelength expressed as reciprocal centimetres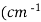 ).
).
The energy associated with electromagnetic radiations is given by,
E= hv = 
- E = energy of radiations
- H = planks constant
- V = frequency of radiation in hz
- C= velocity of light
Electromagnetic spectrum: -
- The electromagnetic spectrum consists of the arrangement of all types of electromagnetic radiations in the order of their increasing wavelength and decreasing frequencies.
2.3.2 Laws of Spectrometry (Lamberts and Beer-Lambert’s law)
UV and visible radiation are energetic electromagnetic radiations that bring about electronic excitations.
The electromagnetic radiations with weakness range 10 – 400nm are called ultraviolet radiation and 400nm are called visible light radiations.
Lambert – beer laws of absorption
Lamberts law: -
The rate of decrease in intensity of radiation is directly proportional to path length of solution when the monochromatic light passes through a solution of constant concentration.
Mathematically the law can be written as,
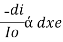
Therefore,
 intensity (radiant power) of the incident radiation.
intensity (radiant power) of the incident radiation.
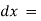 small thickness of solution or path length.
small thickness of solution or path length.
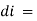 small decrease in intensity of light
small decrease in intensity of light 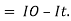
Intensity or radiant power is the number of photons per unit area per second
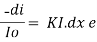
Integrating within limits
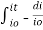 =
= 
Therefore,(ln it – ln io) = ki. x
In io/it = ki.x
Lambert’s law can be stated as the intensity of transmitted light (it) decreases exponentially as the thickness of absorbing medium increase for a fixed concentration.
Beer’s law: -
The rate of decrease in intensity of light proportional to concentration of solution for a fixed thickness of solution.
Mathematically the low can be written as,
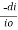 ά dc
ά dc
Integrating within limits
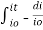 =
= 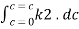
Therefore,
- -( ln it – ln io) = k2.c
- Or ln io/it = k2c
- it = io. e-k2.c
In other words, beers law can be stated as the intensity of transmitted light decrease exponentially with increase of concentration, for a fixed path length of solution.
Combined lambert – beers law
The combined lamberts – beers law will be
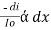 -- --------------------- (lamberts law for constant concentration)
-- --------------------- (lamberts law for constant concentration)
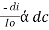 ----------------------- - - (beers law for constant thickness of solution)
----------------------- - - (beers law for constant thickness of solution)
Therefore,
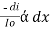 or
or
Integrating within limits
Ln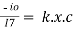
Or
|
Log 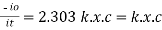
Therefore,
K is absorptivity constant
The term log 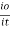 is defined as absorbance (a) and transmittance (T) is defined as it/io
is defined as absorbance (a) and transmittance (T) is defined as it/io
A= K.X.C Lambert – beers law
Therefore,
X is in om C is in gm/lit
Lambert-Beer’s law can be stated as absorption of a light by solution is directly proportional to the concentration of solution and the path length.
By taking unit concentration of sample in solution (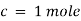 ) and unit length of path (
) and unit length of path (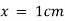 ) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.
) the absorbance observed will be the value of the constant in Lambert – Beer’s equation.
A= E.X.C
Therefore,
E is known as molar absorptive or molar extinction coefficient.
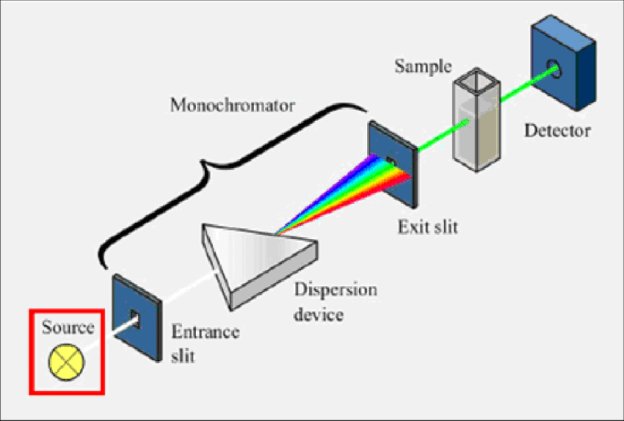
2.3.3 Single beam spectrometer (Schematic, working and applications)
It consists of following parts: -
Source of radiation: It should provide sufficient intensity over the wavelength region where absorption is to be measured for obtaining 300 – 1000 nm wavelength, an in candescent filament, lamp is used, hydrogen or discharge lamp is used.
Mono chromate: A monochromator consists of entrance slit, collimator, a grating or prism, exist slit a good mono chromate provides very narrow wavelength bond.
Glass prisms are used for work in the visible region, quartz prisms are used for UV region.
Gratings are of two types:
Transmission grating: It consists of a series of closely spaced parallel grooves on a transparent material a suitable grating for work in UV – visible region has 2500 -5000 lines per cm.
Reflection grating: Are used more commonly it consists of closely spaced grooves on metallic film.
Slits: The slits are used for selecting the desired wavelength from dispersed light by the monochromator slits are placed in the opposite sides of the prisms or grating.
Sample holder: There is special glass tube is also called as cuvette or sample cell which does not absorb the light from uv – visible spectrum and the sample solution is kept in it. e. g: - quartz glass cuvettes are used in 200 – 300nm and fused glass cuvettes are used in 300 - 2500 nm.
Detector: It is also called as transducers which convert electromagnetic radiation in an e- flow due to that photo current is developed which amplified by amplifier and recorded by recorder.
Schematic Diagram:
|
2.4.1 Introduction
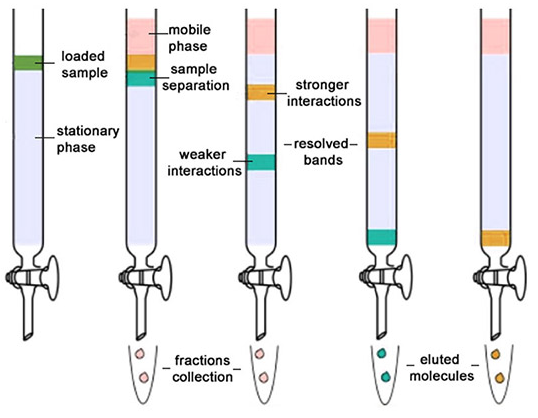
Chromatography is the technique which is used for the separation, purification and testing of the compounds. Chromatography word is derived from the Greek word ‘Chroma’ that means color. Chromatography helps in separating the mixture in its components between in two phases. The stationary phase remains fixed in place while the mobile phase carries the components of the mixture through the medium being used. The stationary phase acts as a constraint on many of the components in a mixture, slowing them down to move slower than the mobile phase. The movement of the components in the mobile phase is controlled by the significance of their interactions with the mobile and stationary phases. Because of the differences in factors such as the solubility of certain components in the mobile phase and the strength of their affinities for the stationary phase, some components will move faster than others, thus facilitating the separation of the components within that mixture.
2.4.2 Types of Chromatography
Chromatography is divided into 4 different parts:
1) Thin Layer Chromatography
2) Adsorption Chromatography
3) Partition Chromatography
4) Column Chromatography
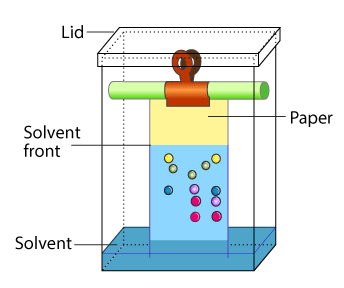
Thin Layer Chromatography: The mixture of substance is separated into its components with the help of glass plate coated with very thin layer of absorbant like silica gel or alumina. The used plate for this purpose is called as the chrome plate. The mixture is applied as a small spot of 2 cm above one end of the plate. The plate is then placed in the closed jar containing a fluid termed as an eluant, which then rises up the plate carrying different components of the mixture to different heights.
|
Adsorption Chromatography: This chromatography is one of the oldest type of chromatography. It utilizes a mobile liquid or gaseous phase that is adsorbed onto the surface of a stationary solid phase. The equilibrium between the mobile and stationary phase accounts for the separation of different solutes.
|
Partition Chromatography: Partition chromatography is based on the continuous differential partition of components of a mixture between stationary and mobile phases. Paper chromatography is a type of partition chromatography. In the paper chromatography a special quality paper is used that is called as the chromatography paper. Chromatography paper contains water trapped in it, which acts as the stationary phase.
|
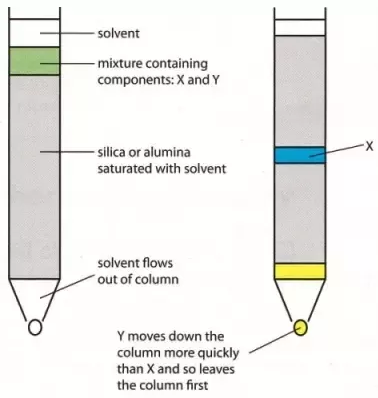
Column Chromatography: Column chromatography is the technique used to separate the components of a mixture using a column of suitable adsorbent packed in a glass tube. The mixture is placed on the top of the column, and an appropriate eluant is made to flow down the column slowly.
Depending upon the degree of adsorption of the components on the wall adsorbent column, the separation of the components takes place. The component with the highest absorptive is retained at the top, while the other flow down to different heights accordingly.
|
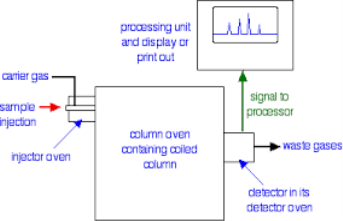
2.4.3 Gas-liquid Chromatograph (GLC):
This is the one of the fastest separation method, which allows for the quantitative determination of each compound of the mixture. In the Gas-Liquid chromatography the process begins by the bringing of sample by the syringe to the heated injection chamber. The sample is passing through the nitrogen gas in the column. The same column is has the thin film of liquid. After the separation of the carried gas the separated mixture flows in detector which activates a recorder. Traces a series of peaks on chromatogram.
|
2.4.4 Basic Principle:
Chromatography is based on the principle where molecules in mixture applied onto the surface or into the solid, and fluid stationary phase is separating from each other while moving with the aid of a mobile phase. The factors effective on this separation process include molecular characteristics related to adsorption (liquid-solid), partition (liquid-solid), and affinity or differences among their molecular weights because of these differences, some components of the mixture stay longer in the stationary phase, and they move slowly in the chromatography system, while others pass rapidly into the mobile phase and leave the system faster.
2.4.5 Instrumentation and Applications
1) The chromatographic technique is used for the separation of amino acids, proteins and carbohydrates.
2) It is helpful for the qualitative and quantitative analysis of complex mixtures.
3) It is also used for the analysis of drugs, hormones, vitamins and brain amines.
4) This technique is also helpful for the determination of molecular weight of proteins.
5) It is helpful in detection of phenolic compounds in drinking water.
6) It is helpful in detection of endogenous Neuro peptides in extracellular fluid of brain etc.