UNIT 1
Introduction to Civil Engineering And Civil Engineering Materials
The main scope of civil engineering or the task of civil engineering is planning, designing, estimating, supervising construction, managing construction, execution, and maintenance of structures like building, roads, bridges, dams, etc
- To carry out planning of building as per its functional needs, as suggested by clients or user, the building may be residential building, public building, or industrial building. He has to plan the building as per the byelaws.
- To carry out soil investigations for the design of foundation of structures.
- To carry out design of structures as per the principles of structural analysis and design. He should also ensure that the design is safe, durable, and economical.
- To prepare the estimates to know the probable cost of completion of work. • To invite tenders & to select contractors for the works.
- To carry out valuation of land or building for the purpose of finding its sale or purchase price or taxation.
- Civil engineers has to work for the general welfare of people.
There are four kinds of measurements used in plane surveying:
1. Horizontal distance 3. Horizontal angle
2. Vertical distance 4. Vertical angle.
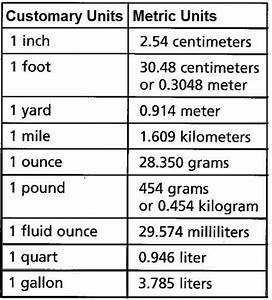
According to the Standards of Weights and Measures Act (India). 1956 the unit of measurement of distance is meters and centimeters. Prior to the introduction of metric units in India, feet, tenths and hundredths of a foot were used.
Types, properties, and uses of some traditional materials used for civil engineering are explained below:
S.No | MATERIAL | TYPES | PROPERTIES | USES |
1 | Cement |
| Chemical properties:
Physical properties:
|
|
2 | Aggregate, |
|
| Used for Construction Works |
3 | Brick, |
|
|
|
4 | Steel,
| Plain carbon steel Alloy steel Low alloy steel Stainless steel | Strength Toughness Ductility Weld ability Durability
| Used as a reinforcement in construction works |
5 | Concrete |
| Workability: Segregation: Bleeding, Good durability Impermeable (e) good resistance to wear and tear. Harshness | 1.As bed concrete below column footings, wall footings, on wall at supports to beams 2. As sill concrete 3. Over the parapet walls as coping concrete 4. For flagging the area around buildings 5. For pavements 6. For making building blocks. |
6 | Stone |
| Resistance to Fire Ease in Dressing: Structure Texture Density
|
|
8
| Mortar | Cement Mortar Lime Mortar Mud Mortar | A mix richer than 1:3 is prone to shrinkage. Well-proportioned mortar provides impervious surface. Plastered surface is Porous. The strength of mortar depends upon the proportion of cement and sand | 1. To bind masonry units like stone, bricks, cement blocks. 2. To plaster slab and walls make them impervious. 3. To give neat finishing to walls and concrete works. 4. For pointing masonry joints. 5. For preparing building blocks. |
9 | Timber | Exogeneous Trees Endogeneous Trees | Texture of good timber is fine and even. In good timber grains are close. Higher the density stronger is the timber. Harder timbers are strong | 1.heavy construction works like columns, trusses, piles. 2. For light construction works like doors, windows, flooring and roofing. 3. For railway sleepers, fencing poles, electric poles and gates. 4.scaffolding, centering, shoring and strutting |
10 | Plastic | Thermosetting and Thermoplastic. | 1.Using pigments plastics of any attractive Color can be produced. 2. Dimensional Stability 3. Durability 4. Electrical Insulation. 5. Fire Resistance | 1. Corrugated and plain sheets for roofing. 2. For making joint less flooring. 3. Flooring tiles. 4. Overhead water tanks. 5. Bath and sink units. 6. Cistern hall floats |
11 | Epoxy | Glycidyl epoxy Non-glycidyl epoxy | A milky white liquid appearance | Used in paint industry |
12 | Fly Ash | Class C type Class F type
| Silt sized particles (spherical shape) | Used in construction works |
13 | Steel Slag | Blast furnace slag Steel furnace slag | Highly angular in shape Rough surface texture | Used as an aggregate |
14 | Copper Slag | Air cooled copper slopper slagag Granulated copper sla | Specific Gravity=4.2 Bulk density=2.31g/cc | Used for surface blast cleaning |
15 | Bitumen | Penetratioied bitumenn grade bitumen Polymer modif | Adhesion resistance to water Hardness Viscosity and flow | Construction of roads Preserving of timber
|
16 | Optical fiber | Single mode O F Multimode O F | Very low attenuation Noise immunity High bandwidth | Used in communication Fiber optics sensor Illumination |
17 | Pipe | Cast iron pipe Galvanized iron pipe Wrought iron pipe etc. | Biological resistance Fire resistance Non inflammable | Used in piping system In the field of Agriculture |
18 | Wire | Solid core wire Stranded wire | High strength Low stiffness High range
| Small and big industries Distribution lines |
19 | Cable | Type NM cable Type MC armored cable | High formability Ultimate tensile strength
| Transmission Lines |
(According to IS code 10500:2012)
Drinking Water — Drinking water is water intended for human consumption for drinking and cooking purposes from any source. It includes water (treated or untreated) supplied by any means for human consumption
Physical Characteristics of Water
1. Turbidity of Water
The turbidity is measured by a turbidity rod or by a turbidity meter with optical observations and is expressed as the amount of suspended matter in mg/l or parts per million (ppm).
. 2. Colour
The presence of colour in water is not objectionable from health point of view, but may spoil the colour of the clothes being washed. The standard unit of colour is that which is produced by one milligram of platinum cobalt dissolved in one litre of distilled water.
For public supplies, the colour number on cobalt scale should not exceed 20 and should be preferably less than 10.
Colour determined by an instrument is known as tintometer.
3. Taste and Odour
The extent of taste or odour present in a particular sample of water is measured by a term called odour intensity, which is related with the threshold odour or threshold odour number.
Water to be tested is therefore gradually diluted with odour free water, and the mixture at which the detection of odour by human observation is just lost, is determined. The number of times the sample is diluted represents the threshold odour number.
For public supplies, the water should generally free from odour, i.e. the threshold number should be 1 and should never exceed 3.
4. Temperature of Water
For potable water, temperature of about about 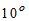 C is desirable. It should not be more than
C is desirable. It should not be more than 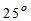 C.
C.
5. Specific Conductivity
The total amount of dissolved salts present in water can be easily estimated by measuring the specific conductivity of water.
Chemical Characteristics of Water
1. Total Solids and Suspended Solids
Total solids (suspended solids + dissolved solids) can be obtained by evaporating a sample of water and weighing the dry residue left and weighing the residue left on the filter paper.
The suspended solid can be found by filtering the water sample. Total permissible amount of solids in water is generally limited to 500 ppm.
2. PH value of Water
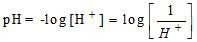
If 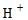 concentration increases, pH decreases and then it will be acidic.
concentration increases, pH decreases and then it will be acidic.
If 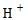 concentration decreases, pH increases and then it will be alkaline.
concentration decreases, pH increases and then it will be alkaline.
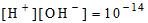
PH + pOH = 14
If the pH of water is more than 7, it will be alkaline and if it is less than 7, it will be acidic.
The alkalinity is caused by the presence of bicarbonate of calcium and magnesium or by the carbonates of hydroxides of sodium, potassium, calcium and magnesium.
Some, but not all of the compounds that cause alkalinity also cause hardness.
3. Hardness of Water
Hard waters are undesirable because they may lead to greater soap consumption, scaling of boilers, causing corrosion and incrustation of pipes, making food tasteless etc.
Temporary Hardness: If bicarbonates and carbonates of calcium and magnesium are present in water, the water is render hard temporarily as this hardness can be removed to some extent by simple boiling or to full extent by adding lime to water. Such a hardness is known as temporary hardness or carbonate hardness.
Permanent Hardness: If sulphates, chlorides and nitrates of calcium or magnesium are present in water, they can not be removed at al by simple boiling and therefore, such water require special treatment for softening. Such a hardness is known as permanent hardness or non-carbonate hardness.
It is caused by sulphates, chlorides, nitrates of Ca and Mg.
4. Chloride Content
The chloride content of treated water to be supplied to the public should not exceed a value of about 250 ppm.
The chloride content of water can be measured by titrating the water with standard silver nitrate solution using potassium chromate as indicator.
5 Nitrogen Content
The presence of nitrogen in water may occur in one or more of the following reasons:
- Free ammonia: It indicates very first stage of decomposition of organic matter. It should not exceed 0.15mg/l
- Albuminous or Organic Matter: It indicates the quantity of nitrogen present in water before the decomposition of organic molten has started. It should not exceed 0.3mg/l
- Nitrites: Not fully oxidized organic matter in water.
- Nitrates: It indicates fully oxidized organic matter in water (representing old pollution).
- Nitrites is highly dangerous and therefore the permissible amount of nitrites in water should be nil.
- Ammonia nitrogen + organic nitrogen = kjeldahl nitrogen
- Nitrates in water is not harmful. However the presence of too much of nitrates in water may adversely affect the health of infants causing a disease called methemoglobinemia commonly called blue baby disease.
- The nitrate concentration in domestic water supplies is limited to 45 mg/l.
6. Metal and other chemical substances in water:
Iron – 0.3ppm, excess of these cause discolouration of clothes.
Manganese – 0.05ppm
Copper – 1.3ppm
Sulphate – 250 ppm
Fluoride – 1.5 ppm, excess of this effects human lungs and other respiratory organs.
Fluoride concentration of less than 0.8 – 1.0 ppm cause dental cavity (tooth decay). If fluoride concentration is greater than 1.5ppm, causing spotting and discolouration of teeth (a disease called fluorosis).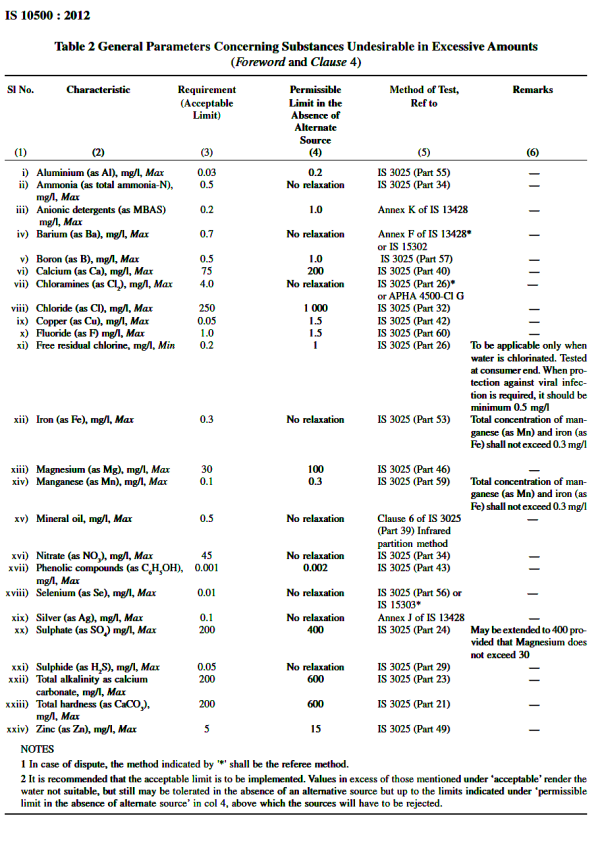
-
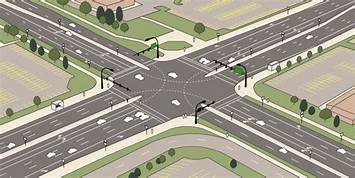
Traffic engineering deals with the measures to be taken for safe, rapid and efficient flow of the traffic. For all this traffic survey should be carried out.
Traffic Survey-This is required to study the type and volume of present traffic and estimate future traffic. It helps in planning expansion or improving the road.
To regulate traffic the following measures are required:
1. Traffic signs
2. Traffic signals
3. Markings.
Traffic Signs-Traffic signs are provided in the form of symbols or inscriptions mounted on fixed or portable supports.
(i) Regulatory signs- These signs are shown on a 600 mm disc installed at a height of 2.8 m above ground level. They may indicate:
• No turn
• No entry
• No parking
• Overtaking prohibited
• Sound horn prohibited, etc.
(ii) Warning signs- These signs are shown on a rectangular board of size 450 × 400 mm mounted at a height of 2.8 m. They may indicate:
• Curve ahead
- Cross road ahead
• Level crossing
• School zones
• U-turns
• Narrow bridge ahead, etc.
(iii) Informatory signs- These are the signs provided to guide drivers to their destination. They may indicate speed limit and name of road also.
These are provided at intersections of roads. They consist of three lights—red to indicate stop, amber to indicate clear and green to indicate ‘go’ signal. In some places the signal indicates how long the red signal will be there. To guide pedestrian, the signals are provided at all
These signal points.
Road Markings-Road markings are necessary to guide road users.
Two types of intersections:
1. Intersection at grades
2. Grade separated intersections
1. Intersections at grades:
• A basic requirement of it is the area of conflict should be small.
• Crossing angles should be preferably at 90° but not less than 60°.
• No blind corners. Any vehicle should safely travel for at least 8 seconds after sighting other
Vehicle.
• Adequate lighting is provided.
• Proper sign, guard rails and traffic islands should be provided.
• Speed should be reduced.
2. Grade separated intersections:
For important roads crossing, flyovers or over crossing may be provided. If major road is above the minor road, it is called over crossing. If minor road is above major road it is called flyover. For entry and exit from major road diamond junction, clover leaf junctions or rotary junctions are provided.