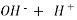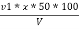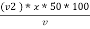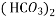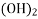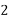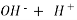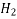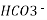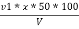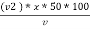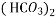UNIT – I
WATER
Water is a chemical compound consisting of two hydrogen atoms and one Oxygen atom. The name water typically refers to the liquid state of the compound. The solid phase is called as ice and the gas phase is called as steam. Under specific conditions, water also forms a supercritical fluid. Water is the main compound found in living organisms. Approximately 62 percent of the human : body contains water. The word "water" comes from the Old English word water or from the Proto-Germanic watar or German Wasser. All of which mean "water" or "wet." The boiling point of water is 99.98 degrees C (211.96 degrees F; 373.13 K).
Water is amphoteric. Which means, it can act as both an acid and as a base.
Hard and soft water:
Hard water: Hard Water is water that contains an required quantity of dissolved minerals (like calcium and magnesium) As rainwater falls, it is naturally soft. However, as water flows through the ground and into waterways, it picks up minerals like chalk, lime and mostly calcium and magnesium and becomes hard water. Since hard water contains essential minerals, it is essentially used as drinking water. Not only because of the health benefits, but also the flavour. Water that does not produce lather with soap readily is called hard water. Water hardness is usually measured as calcium hardness in milligrams per litre (mg/l) OR parts per million (ppm) OR in grains per gallon (GPG).
For e.g.: sea water, river water, spring water, lake water and well water.
Soft water: water that shows the absence of dissolved salts of such metals as magnesium, iron, or calcium, which are known to form insoluble deposits such as appear as scale in boilers or soap curds in bathtubs and laundry equipment, soft water is neither healthy nor desirable to drink. . water that readily produces lather with soap is called soft water.
For e.g.: Rain water, distilled water, demineralised water.
Hardness can be defined as a soap consuming capacity of water sample. soaps are sodium salts of fatty acids like oleic acid, palmetic acid and stearic acid. they dissolve readily .in water to form lather due to which it has cleansing property.
(calciumstearate)
|
TYPES OF WATER | HARDNESS |
Soft | 0 – 75 |
Moderately hard | 75 – 150 |
Hard | 150 – 300 |
Very hard | Above 300 |
There are three types of water quality parameters physical, biological and chemical.The Physical parameters of water quality include:
Turbidity:
Turbidity is the cloudiness present in water. It is a measure of the ability of light to pass through water. It is caused by suspended materials such as silt, clay, plankton, organic material, and other particulate materials present in water.
Turbidity in drinking water is aesthetically unacceptable, which makes the water look unappetizing. The impact of turbidity can be summarized in the following points:
It can increase the cost of water treatment for various uses.
The particulates can shelter harmful microorganisms and thereby protect them from the disinfection process.
Suspended materials can clog or damage fish gills, decreasing its resistance to diseases, reducing its growth rates, affecting egg and larval maturing, and affecting the efficiency of fish catching method.
Suspended particles provide a medium of adsorption for heavy metals such as cadium, lead, chromium, mercury, and many hazardous organic pollutants such as polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), and many types pesticides.
The amount of available food is reduced because higher turbidity raises water. Consequently, the concentration of the dissolved oxygen (DO) can be decreased since warm water carries less dissolved oxygen compared to cold water.
Turbidity can be measured by an instrument called Nephelo metricturbidi meter, the instrument expresses turbidity in terms of NTU or TU. A TU is equivalent to 1 mg/L of silica in suspension.
Turbidity that is more than 5 NTU can be visible to the average person while turbidity in muddy water, it exceeds 100 NTU. Groundwater normally has very low turbidity because of the natural filtration that occurs as the water penetrates through the soil.
Temperature
Palatability, viscosity, solubility, odours, and chemical reactions are influenced by temperature. Thereby, the chlorination and sedimentation, processes and biological oxygen demand (BOD) are dependent on temperature.
Colour
Materials decayed from organic matter, such as any, vegetation and inorganic matter namely stones, soil, and rocks impart colour to water, which is objectionable for aesthetic reasons, not for health reasons.
Colour is measured by comparing the water sample with standard colour solutions or coloured glass disks. One unit of colour is equivalent to the colour produced by a 1 mg/L solution of platinum (potassium chloroplatinate (K2PtCl6)).
The colour of a water sample can be reported as follows:
Apparent colour is the entire water sample colour and consists of both dissolved and suspended components colour.
True colour of the water sample is measured after filtering the water sample to remove all suspended particles.
Colour is graded on scale of 0 (clear) to 70 colour units. Pure water is colourless, which is equivalent to 0 colour units.
Taste and odour
Taste and odour in water can be caused by foreign materials such as organic materials, inorganic compounds, or dissolved gasses. These materials arise from natural, domestic, or agricultural sources.
The numerical value of odour or taste is determined quantitatively by measuring a volume of sample A and diluting it with a volume of sample B of an odour-free distilled water so that the odour of the resulting mixture is just detectable at a total mixture volume of 200 ml. The unit of odour or taste is expressed in terms of a threshold number as follows:
TON or TTN = (A + B)/A
where TON is the threshold odour number and TTN is the threshold taste number.
Solids
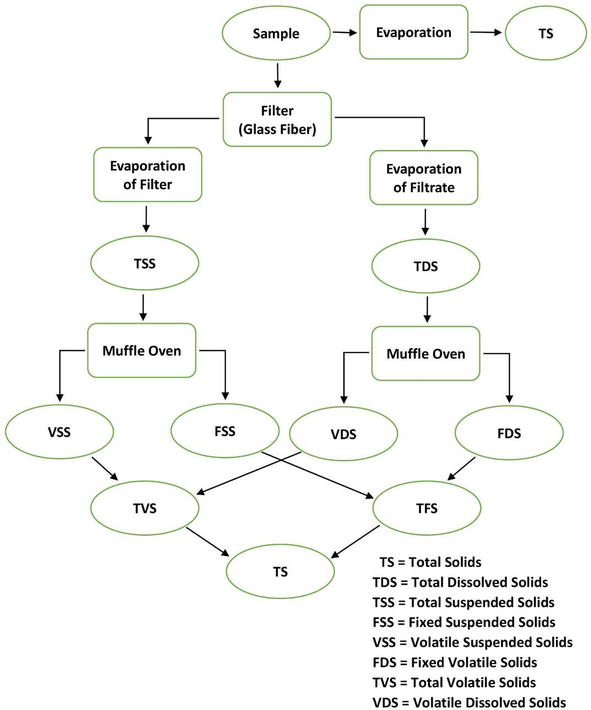
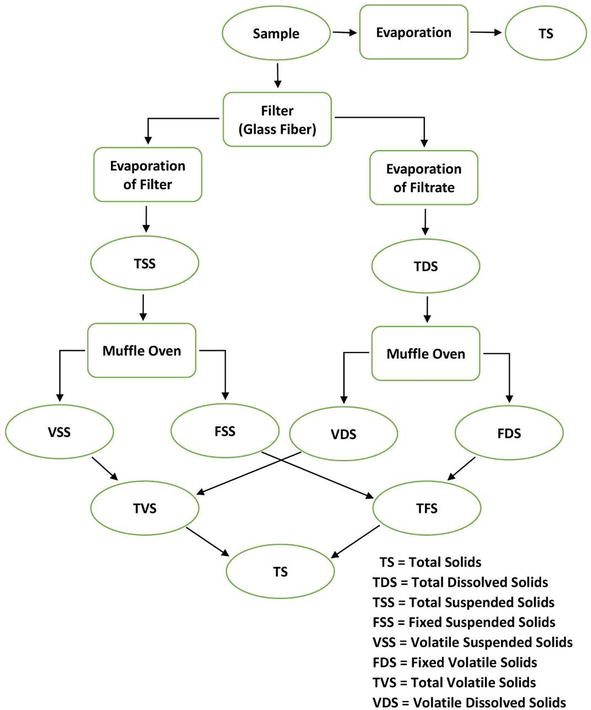
Solids exist in water in either forms, a solution or in suspension. The two types of solids can be identified by using a glass fiber filter through which water sample passes through. By definition, the suspended solids are retained on the top of the filter and the dissolved solids pass through the filter with the water.
when the filtered portion of the water sample is placed in a small dish and then evaporated, the solids form as a residue. This material is called total dissolved solids or TDS.
Total solid (TS) = Total dissolved solid (TDS) + Total suspended solid (TSS)
Water can be classified by the amount of TDS per liter as follows:
freshwater: <1500 mg/L TDS;
brackish water: 1500–5000 mg/L TDS;
saline water: >5000 mg/L TDS.
The residue of TSS and TDS after heating to dryness for a specified period of time and at a specific temperature is defined as fixed solids. Volatile solids are those solids lost on ignition (heating to 550°C).
These necessary measures help the operators of the wastewater treatment plant because they roughly approximate the amount of organic matter that exists in the total solids of Industrialwaste, activated sludge, and wastewater. Fig. 3 describes the interrelationship of solids found in water. They are calculated as follows:
Total solids:
|
Figure 3: Interrelationship of solids found in water.
Total solids (mg/L) = [(TSA – TSB)] × 1000/sample(mL)
The acidity and basicity of the water measured over the pH scale. pH of solution is taken as –ive logarithm of H2 ions for many practical practices. Value range of pH from 7 to 14 is alkaline, from 0 to 7 is acidic and 7 is neutral. Mainly drinking water pH lies from 4.4 to 8.5. The pH scale commonly ranges from 0 to 14.
A natural water may be alkaline due to presence of hydroxide bicarbonates and carbonates compound dissolve in water.
Hydroxides 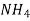 OH, NaOH
OH, NaOH
Bicarbonates Ca (HCO3)2
Carbonates MgCO3 , FeCO3
Hydroxides and carbonates and stronger bases than bicarbonates.
- When an alkaline water is titrated with a strong acid first all OH get neutralized then all the caco3 – ions are half neutralized + OHCo3- .
- Till this stage ,ph of mixture decreases to about 8.2 and completion of this stage is indicated by change in color of phenolphthalein.
- On continued addition of acid during titration all the HCO3 in the titration mixture ( produce by half neutralization of CO3 and present from beginning ) get neutralized and completion of this stage is indicated by methyl orange color change at about3.7 ph.
|
Procedure :-
The alkalineties due to the three type of ions can be easily determined by neutralisation titration.
- Take V ml ( generally 25 ml ) of the alkaline water in conical flask and add 2 drops of phenopthalein indicator in it .
- Titrate this sample against standard strong acid solution ( x n ) from burette till pin k colour changes to colourless . klet the burette be V 1 ml .
- Add few drops of methyl orange indicator into the same titrarting mixture changes to orange .
Note the burette reading as V 2 ml ( from initial )
Calculations :- P = phenolphthalein alkalinity = = PPM Caco3 equivalent M = methyl orange alkalinity = total alkalinity =
|
The possible combinatuions of alkalinites in water are:-
- Only OH-
- Only HCO3-
- ONLY CO3-
- OH- and CO3 – Togther
Chloride ions amount in water sample ( bymoho’s method)
- Under ground and surface water are rich with cl- in the form of Nacl ,Kcl , e.t.c .
- Their quantity over 250 mgl liter imparts bad taste to water. Mgcl2 , Cacl2 cause hardness and being the salts of weak base strong acid are harmful for industry and boiler use .
Theory
- Cl – quantity in a water sample is found precipitation titration method. ( mohor’s method )
 In the mohor’smethod ,
In the mohor’smethod ,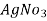 is the titrant and potassium chromate as indicator in the titration.
is the titrant and potassium chromate as indicator in the titration.
 ( Ksp = 1.82 *
( Ksp = 1.82 * 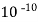 )
)
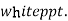

 ( ksp = 1.1 *
( ksp = 1.1 * 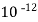 )
)
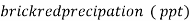

3. When Ag + ions are added to the mixture container of an indicator Cro4 there is first formation of Agcl , although Agcl has lesser solubility product than Ag2Cro4.
4. The reason for why Ag2Cro4 precipitate formation requires excess concentration of Ag++ to exceed its ksp.
Procedure :-
- Take 50 ml of a chloride water sample in a conical flask and add a pinch of caco3 to neutralize the water if it is acidic.
- Add few drops of a potassium chromatic indicator solution into the water and slowly add Agno3 solution( z molarity ) from burette.
- Note the end point when permanent reddish tinge is obtained .let the burette reading by Y ml .
Calculations :-
 volume of
volume of 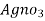 for 50 ml water sample = Y ml
for 50 ml water sample = Y ml
∴volume of 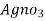 for 1000 ml water sample 1000/50 * y ml
for 1000 ml water sample 1000/50 * y ml
= 20 y ml
∴ 1000 ml 1 m 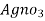 = 35.5 * 1000 mg cl
= 35.5 * 1000 mg cl
∴ 20 y ml 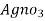 =
= 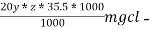
= 20yz * 35.5 mg cl- / litre
Adequate dissolved oxygen concentrations are critical during all phases of striped bass and hybrid culture. Low dissolved oxygen concentrations can result in slower growth and induce the stress response predisposing the animals to infectious disease. Monitoring of dissolved oxygen concentrations is complicated by the rate at which they can change. In heavily stocked raceways, tanks, or flow-through systems, for example, an interruption of oxygenation may result in critically low dissolved oxygen concentrations within minutes due to consumption by the culture animals. Management of dissolved oxygen concentrations in ponds must also consider the daily rhythms of concentrations characteristic of ponds. Striped bass and its hybrids have different dissolved oxygen requirements at different stages in their lives. Striped bass also appear to require higher concentrations of dissolved oxygen relative to other temperate species. Generally, dissolved oxygen concentrations should be maintained as close to saturation as possible for best survival and growth.
Hardness can be defined as a soap consuming capacity of water sample. soaps are sodium salts of fatty acids like oleic acid, palmetic acid and stearic acid. they dissolve readily .in water to form lather due to which it has cleansing property.
|
- But compounds of fatty acids with other metals done dissolve in water.
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd.
|
(calciumstearate)
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd
- These other metal ions are responsible for the hardness of water most important metal of ions which cause hardness to water are calcium and magnesium ions.
- The hardness of water along can be calculated from the amount of calcium and magnesium ions present in water along with bicarbonates, sulphates chlorides and nitrates.
TYPES OF WATER | HARDNESS |
Soft | 0 – 75 |
Moderately hard | 75 – 150 |
Hard | 150 – 300 |
Very hard | Above 300 |
Types of hardness :
- Temporary hardness ( carbonate) :-
- When water containing calcium and magnesium bicarbonates is heated , bicarbonate decompose and from insoluble carbonate and form hydroxide.
- On filtering such water , soft water is obtained.
- The hardness which can be removed by more boiling is referred as ‘temporary hardness ’ or bicorbonate hardness.

Mg ( Bicarbonates)
|
II. Permanent hardness :-
- The term permanent hardness ornon carbonate is the term applied to the hardness caused by dissolved chlorides , nitrates and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- Sum of temporary and permanent hardness is referred to as total hardness.
III. Alkaline or carbonate and non – alkaline or non – carbonate hardness :-
- Like all carbonate and bicarbonate , calcium and magnesium carbonate and bicarbonate are alkaline.
- Then hardness due to the carbonate and bicarbonate is called alkaline hardness or carbonate hardness.
- The alkalinity can be measures by titration with standard mineral acid using methyl orange and or phenol phthalein as an indicator.
4. As the sulphate and chloride are neutral salts , the hardness caused by presence of calcium and magnesium sulphate , chlorides and nitrates is termed as non alkaline hardness or non carbonate hardness.
IV. Total hardness :-
The hardness due to all hardness causing salts, known as total hardness.
Total hardness = temporary + permanent.
Estimation of hardness :-
Hardness of weather can be determined by two methods.
1) Soap solution method :-
- Total hardness of water can be determined by titrating a fixed volume of water sample (100ml) against standard alcoholic soap solution.
- Appearance of stable lather which persists for two minutes is the end point of titration.
- In the beginning sodium soap will precipitate all the hardness causing metal ions in the form of their soap (card) and then it will form free lather.
- If same water sample is boiled for 30minutes and then titrated against same soap solution the titration reading corresponds to permanent hardness.
- The difference between two measurements corresponds to the temporary hardness of water.
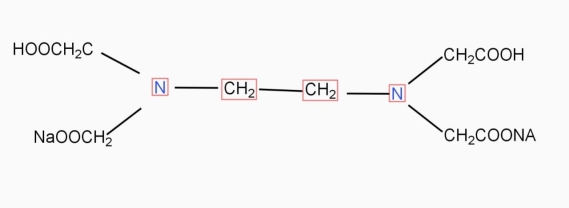
EDTA method :
- Hardness of water can be determined more accurately by EDTA method.
- In this method 100ml of water sample is taken in titration flask to this 3ml of buffer an 08 ph 10 is added.
- Then it is titrated against 0.01m EDTA using electron black T as an indicator.
- At the end point wine red color changes to blue color.
- From barettereading , total hardness of water sample can be calculated using the formula.
1000ml of 1MEDTA = Caco3 = 100 get Caco3
6. Suppose barrette reading is xml for 100 ml of water sample. Then for one liter of sample 10x ml EDTA is required.
As ,
100 ml 1MEDTA = Caco3 = 100 get Caco3
1 ml 0.01 EDTA = 1 mg Caco3
10 x ml 0.01m EDTA = 10 x mg Caco3
7. Hardness can be expressed as mg of Caco3 present in 10 mg ( 1 liter ) of water i.e. ppm .
Hardness of such water sample will be 10 x ppm
8. If titration is carried out after boiling the water sample for 30 min the reading will correspond to permanent hardness corresponds to temporary hardness of water sample.
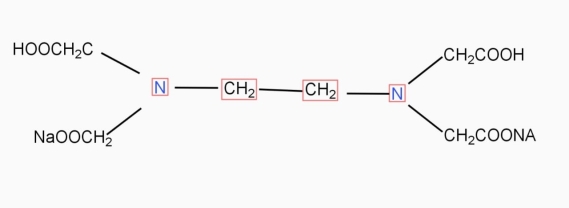
9. Ethylene- diamineteracetic acid (EDTA) IS PRACTICALLY Insoluble in water it is represented as H4Y.
|
IN aqueous solution ionises as
Na2H2Y  2Na+ + H2Y2-
2Na+ + H2Y2-
It forms 1:1 complex with Ca++ and Mg++ metal ions present in water sample when indicators is added to water sample colored ( red ) metal indicator complex is formed
When this is titrated with EDTA solution
H2Y²- ions react with Ca++ or Mg++ ions from metal indicators complex because these two have more affinity towards EDTA. So more stable metal EDTA complex is formed .at the same time HIn² -ve ions oxygenare set free ( blue ).
So, at the end point color changes from red to blue.
- Parts of per million (ppm) is the parts of the calcium carbonate equivalent hardness per 10 raise to 6 parts of water i.e. 1 ppm = 1 part of
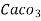 eq hardness in
eq hardness in 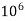 parts of water .
parts of water . - Milligram per liter is the number of milligram of
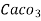 equivalent hardness present per liter of water.
equivalent hardness present per liter of water.
Thus,
1 mg/l = 1 mg of 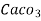 eq. hardness of 1L of water .
eq. hardness of 1L of water .
But 1 L OF Water weighs.
= 1 kg = 1000 g = 1000*1000 mg = 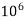 mg
mg
Therefore,
1mg/l = 1 mg of 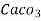 eq. per
eq. per 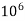 mg of water.
mg of water.
= 1 part of 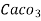 eq. per
eq. per 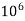 mg of water
mg of water
= 1 part of 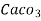 eq. per
eq. per 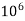 parts of water
parts of water
=1 ppm
3. Clarke’s degree is number of grains (1/7000lb) of 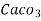 equivalent hardness per gallon (10lb) of water. Or it is parts of
equivalent hardness per gallon (10lb) of water. Or it is parts of 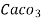 equivalent hardness per 70000 parts of water.
equivalent hardness per 70000 parts of water.
Thus,
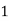 ° clark = 1 grain of
° clark = 1 grain of 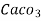 eq. hardness per gallon of water.
eq. hardness per gallon of water.
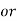 1° c l = 1 part of
1° c l = 1 part of 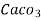 eq. hardness per 70000 parts of water .
eq. hardness per 70000 parts of water .
4. Degree French (°fr) is the part of 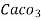 equivalent hardness per
equivalent hardness per 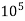 part of water .
part of water .
Thus,
1°fr = 1 part of 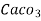 hardness eq. per
hardness eq. per 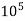 parts of water.
parts of water.
5. Milli equivalent per liter (meq / L )is the number of milli equivalents of hardness present per liter.
Thus,
1meq/L = 1 meq of 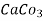 per L of water
per L of water
= 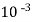 * 50 g of
* 50 g of 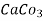 eq. per liter.
eq. per liter.
= 50 mg of 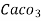 eq per liter.
eq per liter.
= 50 mg / l of 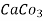 eq = 50 ppm.
eq = 50 ppm.
Relation between various units of hardness
- 1 ppm = 1 mg / L 0.1° fr = 0.07° Cl = 0.02 meq/L
- 1 mg/L = 1 ppm = 0.1° fr = 0.07° cl = 0.02 meq / L
- 1°cl = 1.433° fr = 14.3 ppm = 14.3 mg / L = 0.286 meq / L
- 1° fr = 10 ppm = 10 mg/ L = 0.7° cl = 0.2 meq / L
- 1 meq/ l = 50 mg/ l = 50ppm = 5° fr = 0.35° cl
formation)
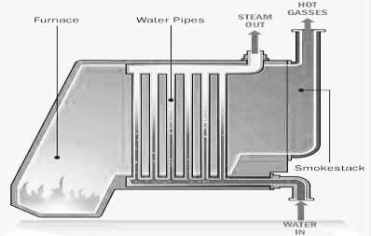
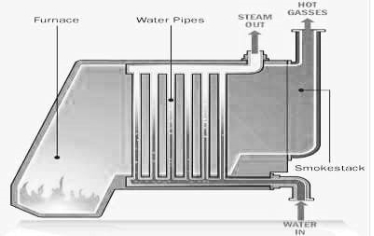
In steam generation and to increase the life of the boiler .
For the purpose the feed water is treated well externally ( by processes like ion exchange , zoolite , soda lime ) and internally ( by processes like phosphate , conditioning , hydrazine , tretament )depending upon the operating pressure of boiler , the feed water should satisfy the following requirement.
Type of boiler | Permitted hardness of feed water |
Low pressure below 15kg/cm² | 25 – 50 ppm caco3 equivalent |
Medium pressure 15 – 30 kg/cm² | 10 – 25 ppm caco3 equivalent |
High pressure greater than 30 kg/cm² | 0 – 10 ppm caco3 equivalent |
- The most important use of water as enginnering material is the steam generation .
- Steam is required for power generation in thermal power stations for uniform and controlled heating of reactors for steam engine e.t.c .
- Different design of boilers ( water tube, vertical , e.t.c ) are used in making steam of different pressure and temprature.
- Water of minimum hardness 9 below 10 ppm is used for high pressure steam boilers ) and maximum purity ( total dissolved solids below 25 mg / lit) is good for steam generation .
- Water of higher hardness and other imparites is treated well before . fedding it in boiler.
|
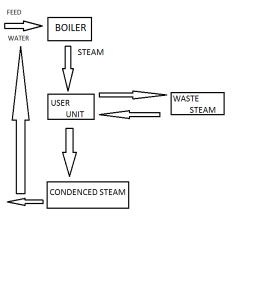
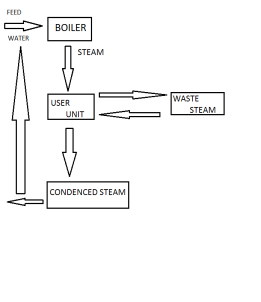
Steam generation can be economized by recircuit calculating the waste steam on reheating and recircualting the steam condensalte to boiler from the steam generation.
|
Major boiler problems due to use of unsutiable water are :-
- Corrison
- Priming and foaming
- Sludges and scales formation
- Caustic embittlement
Out of these boiler troubles the corrison and seals formation are much serve and requires atmost care.
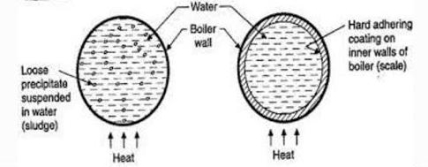
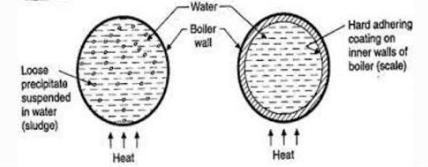
Scale and Sludge Formation:
- In boiler water evaporates continously and the concentration of salts left behind goes on increasing . After the saturation point they get precipitated.
- If the precipitate remains in boiler tube as loose and slimy matter is called sludge.
- If some of the precipitated matter adhers strongly and forms strong bad conducting layer on their inner side of boiler tube , then it is known as scale.
Scale
A] formation of scales :-
Scale is the hard and strongly adhered coating to the inner surface of boiler and it is a bad conductor of heat. It is the main source of boiler trouble.
It is caused due to :-
- Decomposition of bicarbonates :-
At high temprature bicarbonates decompose into sticky water insolube material.
Ca ( HcO3)2 ----------------- CaCO3 + H2O + CO2
Mg ( HCo3)2 - - -------------- Mg (Oh)2 + 2CO2
2. Hydrolsis of magnesium salts :-
At higher temprature magnesium salts undergo hydrolysis.
MgCl2 + 2H2O ---------------------- mg ( OH)2 + 2HCl
3. Presence of silica :-
The source of ssilica is ( form) from sand and filter .silica may be in the form of colloidal particles. And it can be deposite as calcium silicate or magnesium silicate as firmly adhering materials.
4. Decreased solublity of CaSO4 :-
CaSo4 has lesser solublity at higher temprature hence at high temprature CaSo4 present in boiler feed water will precipitate as hard scale forming materials.
|
Sludge
Formation of sludge
- The loose slimy mass of salts precipitated in boiler water is the sludge.
- They are generally formed at cooler portion of boiler and they loosely deposit in the parts of boiler tube where flow rate is slow e.g vlves bends
- Sludges are easy to remove by using brushes detergent solutions blow down opreation e.t.c .
Formula :-
Hardness of water by EDTA
1) When standard hard water is used
Total hardness of water sample = 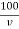 .
. 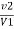 . Mg. CaCo3
. Mg. CaCo3
Where,
M = mgCaco3 in titrated standard hard water
V1 = EDTA volume for titrated standard hard water
V2 = EDTA volume for V ml water sample
2) When EDTA of known molarity used
Total hardness of water sample =  *z*
*z*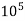 MgCo3
MgCo3
Where,
Y = EDTA volume for V ml of water sample
Z = molarity of EDTA solution
Chloride ions in water sample :-
cl- quantity = 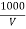 yz (35.5) mgel/L
yz (35.5) mgel/L
Where,
Y = volume of AgNo3
Z = molarity of AgNo3
Alkalinites in water sample
Phenolphthalien alkalinty
P = 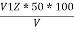 PPM Caco3 equivalent
PPM Caco3 equivalent
Methyl orange alkalinity
M = 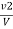 * z * 50 * 1000 ppm
* z * 50 * 1000 ppm
Where ,
V1 = volume of acid for V ml water sample in titraton with use of phenolphthalein indicator.
V2 = volume of acid for V ml water sample in the continued titration using methyl orange indicator.
Z = normally of acid in barette.
Ion exchange process:
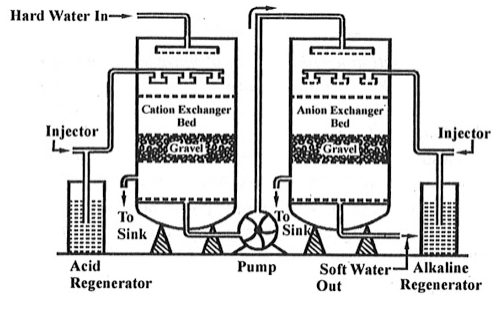
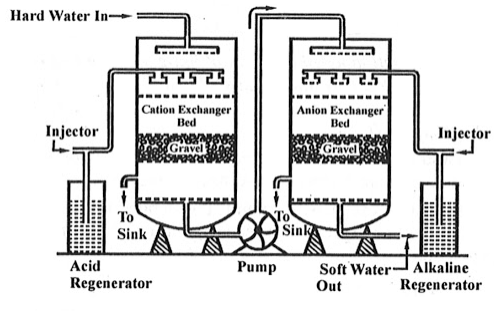
Ion exchange technology is a proven method of producing high purity softened and demineralized water. It is used in most industries that require high purity water and to reclaim water from processes. The Ion exchange process involves the exchanging of contaminant ions for Na+ ions in a softening application and H+ and OH- ions in pure water application. Cations and anions can be removed by the cation and anion exchange resins. Resins containing –COOH, SO3H are capable for exchanging their H+ ions to cationic portion of minerals then it is called as cation exchanger while the resins containing –NH2, NHCH3 are capable for exchanging the anionic portion of the minerals then it is termed as anionic exchanger.
|
On supplying the hard water in first chamber which consists of Ca2+ or Mg2+ then the cation exchanger exchange it with H+ hence the cation exchanger absorbs the Ca2+ ions the left water are free from cations are passed to another chamber by the help of pump this water consists of anions such as Cl or SO4 on sprinkle up of these water at anion exchanger bed then it exchanged the anions and hence release the demineralise water. The absorbed cation and anion are sinked out through the outlet present in chamber.
Reverse osmosis:
Reverse osmosis is a process where the water is separated from the salts in the source water by pressure-driven transport through a membrane. This process uses semi-permeable membrane and applied pressure to preferentially induce water permeation through the membrane while rejecting salts. The RO plant uses less energy than thermal desalination process. This process uses thin-film composite membrane that too comprises of ultra thin aromatic polyamide thin film. The used polyamide film gives the transparent properties while the remaining part provides the mechanical supports. The polyamide films are very dense void free polymer with high surface area allowing for its high permeability. RO desalination plant include source water intake system, pre treatment facilities and high pressure feed pumps, RO membrane trains, energy recovery and desalinated water conditioning system. The intake system may be the open surface water intake or series of seawater beach wells. The pretreatment system may be the screening, chemical conditioning, sedimentation or filtration that totally depends on the used quality water further the filtered water is conveyed by transfer pump from filtrate water storage tank through cartridge filter and into the suction pipe of high pressure RO feed pumps. The cartridge filters are designed in such a manner that can retain 1 to 20 microns particles which remained in the source water after pretreatment. The high pressure feed pumps are designed to deliver the source water to the RO membranes at pressure required for membrane separation of the fresh water from the salts. The actual required feed pressure is site-specific and is mainly determined by the source water salinity and the configuration of the RO system.
UNIT – I
WATER
Water is a chemical compound consisting of two hydrogen atoms and one Oxygen atom. The name water typically refers to the liquid state of the compound. The solid phase is called as ice and the gas phase is called as steam. Under specific conditions, water also forms a supercritical fluid. Water is the main compound found in living organisms. Approximately 62 percent of the human : body contains water. The word "water" comes from the Old English word water or from the Proto-Germanic watar or German Wasser. All of which mean "water" or "wet." The boiling point of water is 99.98 degrees C (211.96 degrees F; 373.13 K).
Water is amphoteric. Which means, it can act as both an acid and as a base.
Hard and soft water:
Hard water: Hard Water is water that contains an required quantity of dissolved minerals (like calcium and magnesium) As rainwater falls, it is naturally soft. However, as water flows through the ground and into waterways, it picks up minerals like chalk, lime and mostly calcium and magnesium and becomes hard water. Since hard water contains essential minerals, it is essentially used as drinking water. Not only because of the health benefits, but also the flavour. Water that does not produce lather with soap readily is called hard water. Water hardness is usually measured as calcium hardness in milligrams per litre (mg/l) OR parts per million (ppm) OR in grains per gallon (GPG).
For e.g.: sea water, river water, spring water, lake water and well water.
Soft water: water that shows the absence of dissolved salts of such metals as magnesium, iron, or calcium, which are known to form insoluble deposits such as appear as scale in boilers or soap curds in bathtubs and laundry equipment, soft water is neither healthy nor desirable to drink. . water that readily produces lather with soap is called soft water.
For e.g.: Rain water, distilled water, demineralised water.
Hardness can be defined as a soap consuming capacity of water sample. soaps are sodium salts of fatty acids like oleic acid, palmetic acid and stearic acid. they dissolve readily .in water to form lather due to which it has cleansing property.
(calciumstearate)
|
TYPES OF WATER | HARDNESS |
Soft | 0 – 75 |
Moderately hard | 75 – 150 |
Hard | 150 – 300 |
Very hard | Above 300 |
There are three types of water quality parameters physical, biological and chemical.The Physical parameters of water quality include:
Turbidity:
Turbidity is the cloudiness present in water. It is a measure of the ability of light to pass through water. It is caused by suspended materials such as silt, clay, plankton, organic material, and other particulate materials present in water.
Turbidity in drinking water is aesthetically unacceptable, which makes the water look unappetizing. The impact of turbidity can be summarized in the following points:
It can increase the cost of water treatment for various uses.
The particulates can shelter harmful microorganisms and thereby protect them from the disinfection process.
Suspended materials can clog or damage fish gills, decreasing its resistance to diseases, reducing its growth rates, affecting egg and larval maturing, and affecting the efficiency of fish catching method.
Suspended particles provide a medium of adsorption for heavy metals such as cadium, lead, chromium, mercury, and many hazardous organic pollutants such as polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), and many types pesticides.
The amount of available food is reduced because higher turbidity raises water. Consequently, the concentration of the dissolved oxygen (DO) can be decreased since warm water carries less dissolved oxygen compared to cold water.
Turbidity can be measured by an instrument called Nephelo metricturbidi meter, the instrument expresses turbidity in terms of NTU or TU. A TU is equivalent to 1 mg/L of silica in suspension.
Turbidity that is more than 5 NTU can be visible to the average person while turbidity in muddy water, it exceeds 100 NTU. Groundwater normally has very low turbidity because of the natural filtration that occurs as the water penetrates through the soil.
Temperature
Palatability, viscosity, solubility, odours, and chemical reactions are influenced by temperature. Thereby, the chlorination and sedimentation, processes and biological oxygen demand (BOD) are dependent on temperature.
Colour
Materials decayed from organic matter, such as any, vegetation and inorganic matter namely stones, soil, and rocks impart colour to water, which is objectionable for aesthetic reasons, not for health reasons.
Colour is measured by comparing the water sample with standard colour solutions or coloured glass disks. One unit of colour is equivalent to the colour produced by a 1 mg/L solution of platinum (potassium chloroplatinate (K2PtCl6)).
The colour of a water sample can be reported as follows:
Apparent colour is the entire water sample colour and consists of both dissolved and suspended components colour.
True colour of the water sample is measured after filtering the water sample to remove all suspended particles.
Colour is graded on scale of 0 (clear) to 70 colour units. Pure water is colourless, which is equivalent to 0 colour units.
Taste and odour
Taste and odour in water can be caused by foreign materials such as organic materials, inorganic compounds, or dissolved gasses. These materials arise from natural, domestic, or agricultural sources.
The numerical value of odour or taste is determined quantitatively by measuring a volume of sample A and diluting it with a volume of sample B of an odour-free distilled water so that the odour of the resulting mixture is just detectable at a total mixture volume of 200 ml. The unit of odour or taste is expressed in terms of a threshold number as follows:
TON or TTN = (A + B)/A
where TON is the threshold odour number and TTN is the threshold taste number.
Solids
Solids exist in water in either forms, a solution or in suspension. The two types of solids can be identified by using a glass fiber filter through which water sample passes through. By definition, the suspended solids are retained on the top of the filter and the dissolved solids pass through the filter with the water.
when the filtered portion of the water sample is placed in a small dish and then evaporated, the solids form as a residue. This material is called total dissolved solids or TDS.
Total solid (TS) = Total dissolved solid (TDS) + Total suspended solid (TSS)
Water can be classified by the amount of TDS per liter as follows:
freshwater: <1500 mg/L TDS;
brackish water: 1500–5000 mg/L TDS;
saline water: >5000 mg/L TDS.
The residue of TSS and TDS after heating to dryness for a specified period of time and at a specific temperature is defined as fixed solids. Volatile solids are those solids lost on ignition (heating to 550°C).
These necessary measures help the operators of the wastewater treatment plant because they roughly approximate the amount of organic matter that exists in the total solids of Industrialwaste, activated sludge, and wastewater. Fig. 3 describes the interrelationship of solids found in water. They are calculated as follows:
Total solids:
|
Figure 3: Interrelationship of solids found in water.
Total solids (mg/L) = [(TSA – TSB)] × 1000/sample(mL)
The acidity and basicity of the water measured over the pH scale. pH of solution is taken as –ive logarithm of H2 ions for many practical practices. Value range of pH from 7 to 14 is alkaline, from 0 to 7 is acidic and 7 is neutral. Mainly drinking water pH lies from 4.4 to 8.5. The pH scale commonly ranges from 0 to 14.
A natural water may be alkaline due to presence of hydroxide bicarbonates and carbonates compound dissolve in water.
Hydroxides 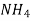 OH, NaOH
OH, NaOH
Bicarbonates Ca (HCO3)2
Carbonates MgCO3 , FeCO3
Hydroxides and carbonates and stronger bases than bicarbonates.
- When an alkaline water is titrated with a strong acid first all OH get neutralized then all the caco3 – ions are half neutralized + OHCo3- .
- Till this stage ,ph of mixture decreases to about 8.2 and completion of this stage is indicated by change in color of phenolphthalein.
- On continued addition of acid during titration all the HCO3 in the titration mixture ( produce by half neutralization of CO3 and present from beginning ) get neutralized and completion of this stage is indicated by methyl orange color change at about3.7 ph.
|
Procedure :-
The alkalineties due to the three type of ions can be easily determined by neutralisation titration.
- Take V ml ( generally 25 ml ) of the alkaline water in conical flask and add 2 drops of phenopthalein indicator in it .
- Titrate this sample against standard strong acid solution ( x n ) from burette till pin k colour changes to colourless . klet the burette be V 1 ml .
- Add few drops of methyl orange indicator into the same titrarting mixture changes to orange .
Note the burette reading as V 2 ml ( from initial )
Calculations :- P = phenolphthalein alkalinity = = PPM Caco3 equivalent M = methyl orange alkalinity = total alkalinity =
|
The possible combinatuions of alkalinites in water are:-
- Only OH-
- Only HCO3-
- ONLY CO3-
- OH- and CO3 – Togther
Chloride ions amount in water sample ( bymoho’s method)
- Under ground and surface water are rich with cl- in the form of Nacl ,Kcl , e.t.c .
- Their quantity over 250 mgl liter imparts bad taste to water. Mgcl2 , Cacl2 cause hardness and being the salts of weak base strong acid are harmful for industry and boiler use .
Theory
- Cl – quantity in a water sample is found precipitation titration method. ( mohor’s method )
 In the mohor’smethod ,
In the mohor’smethod ,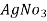 is the titrant and potassium chromate as indicator in the titration.
is the titrant and potassium chromate as indicator in the titration.
 ( Ksp = 1.82 *
( Ksp = 1.82 * 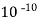 )
)
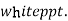

 ( ksp = 1.1 *
( ksp = 1.1 * 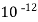 )
)
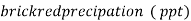

3. When Ag + ions are added to the mixture container of an indicator Cro4 there is first formation of Agcl , although Agcl has lesser solubility product than Ag2Cro4.
4. The reason for why Ag2Cro4 precipitate formation requires excess concentration of Ag++ to exceed its ksp.
Procedure :-
- Take 50 ml of a chloride water sample in a conical flask and add a pinch of caco3 to neutralize the water if it is acidic.
- Add few drops of a potassium chromatic indicator solution into the water and slowly add Agno3 solution( z molarity ) from burette.
- Note the end point when permanent reddish tinge is obtained .let the burette reading by Y ml .
Calculations :-
 volume of
volume of 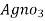 for 50 ml water sample = Y ml
for 50 ml water sample = Y ml
∴volume of 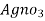 for 1000 ml water sample 1000/50 * y ml
for 1000 ml water sample 1000/50 * y ml
= 20 y ml
∴ 1000 ml 1 m 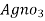 = 35.5 * 1000 mg cl
= 35.5 * 1000 mg cl
∴ 20 y ml 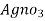 =
= 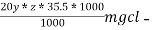
= 20yz * 35.5 mg cl- / litre
Adequate dissolved oxygen concentrations are critical during all phases of striped bass and hybrid culture. Low dissolved oxygen concentrations can result in slower growth and induce the stress response predisposing the animals to infectious disease. Monitoring of dissolved oxygen concentrations is complicated by the rate at which they can change. In heavily stocked raceways, tanks, or flow-through systems, for example, an interruption of oxygenation may result in critically low dissolved oxygen concentrations within minutes due to consumption by the culture animals. Management of dissolved oxygen concentrations in ponds must also consider the daily rhythms of concentrations characteristic of ponds. Striped bass and its hybrids have different dissolved oxygen requirements at different stages in their lives. Striped bass also appear to require higher concentrations of dissolved oxygen relative to other temperate species. Generally, dissolved oxygen concentrations should be maintained as close to saturation as possible for best survival and growth.
Hardness can be defined as a soap consuming capacity of water sample. soaps are sodium salts of fatty acids like oleic acid, palmetic acid and stearic acid. they dissolve readily .in water to form lather due to which it has cleansing property.
|
- But compounds of fatty acids with other metals done dissolve in water.
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd.
|
(calciumstearate)
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd
- These other metal ions are responsible for the hardness of water most important metal of ions which cause hardness to water are calcium and magnesium ions.
- The hardness of water along can be calculated from the amount of calcium and magnesium ions present in water along with bicarbonates, sulphates chlorides and nitrates.
TYPES OF WATER | HARDNESS |
Soft | 0 – 75 |
Moderately hard | 75 – 150 |
Hard | 150 – 300 |
Very hard | Above 300 |
Types of hardness :
- Temporary hardness ( carbonate) :-
- When water containing calcium and magnesium bicarbonates is heated , bicarbonate decompose and from insoluble carbonate and form hydroxide.
- On filtering such water , soft water is obtained.
- The hardness which can be removed by more boiling is referred as ‘temporary hardness ’ or bicorbonate hardness.

Mg ( Bicarbonates)
|
II. Permanent hardness :-
- The term permanent hardness ornon carbonate is the term applied to the hardness caused by dissolved chlorides , nitrates and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- Sum of temporary and permanent hardness is referred to as total hardness.
III. Alkaline or carbonate and non – alkaline or non – carbonate hardness :-
- Like all carbonate and bicarbonate , calcium and magnesium carbonate and bicarbonate are alkaline.
- Then hardness due to the carbonate and bicarbonate is called alkaline hardness or carbonate hardness.
- The alkalinity can be measures by titration with standard mineral acid using methyl orange and or phenol phthalein as an indicator.
4. As the sulphate and chloride are neutral salts , the hardness caused by presence of calcium and magnesium sulphate , chlorides and nitrates is termed as non alkaline hardness or non carbonate hardness.
IV. Total hardness :-
The hardness due to all hardness causing salts, known as total hardness.
Total hardness = temporary + permanent.
Estimation of hardness :-
Hardness of weather can be determined by two methods.
1) Soap solution method :-
- Total hardness of water can be determined by titrating a fixed volume of water sample (100ml) against standard alcoholic soap solution.
- Appearance of stable lather which persists for two minutes is the end point of titration.
- In the beginning sodium soap will precipitate all the hardness causing metal ions in the form of their soap (card) and then it will form free lather.
- If same water sample is boiled for 30minutes and then titrated against same soap solution the titration reading corresponds to permanent hardness.
- The difference between two measurements corresponds to the temporary hardness of water.
EDTA method :
- Hardness of water can be determined more accurately by EDTA method.
- In this method 100ml of water sample is taken in titration flask to this 3ml of buffer an 08 ph 10 is added.
- Then it is titrated against 0.01m EDTA using electron black T as an indicator.
- At the end point wine red color changes to blue color.
- From barettereading , total hardness of water sample can be calculated using the formula.
1000ml of 1MEDTA = Caco3 = 100 get Caco3
6. Suppose barrette reading is xml for 100 ml of water sample. Then for one liter of sample 10x ml EDTA is required.
As ,
100 ml 1MEDTA = Caco3 = 100 get Caco3
1 ml 0.01 EDTA = 1 mg Caco3
10 x ml 0.01m EDTA = 10 x mg Caco3
7. Hardness can be expressed as mg of Caco3 present in 10 mg ( 1 liter ) of water i.e. ppm .
Hardness of such water sample will be 10 x ppm
8. If titration is carried out after boiling the water sample for 30 min the reading will correspond to permanent hardness corresponds to temporary hardness of water sample.
9. Ethylene- diamineteracetic acid (EDTA) IS PRACTICALLY Insoluble in water it is represented as H4Y.
|
IN aqueous solution ionises as
Na2H2Y  2Na+ + H2Y2-
2Na+ + H2Y2-
It forms 1:1 complex with Ca++ and Mg++ metal ions present in water sample when indicators is added to water sample colored ( red ) metal indicator complex is formed
When this is titrated with EDTA solution
H2Y²- ions react with Ca++ or Mg++ ions from metal indicators complex because these two have more affinity towards EDTA. So more stable metal EDTA complex is formed .at the same time HIn² -ve ions oxygenare set free ( blue ).
So, at the end point color changes from red to blue.
- Parts of per million (ppm) is the parts of the calcium carbonate equivalent hardness per 10 raise to 6 parts of water i.e. 1 ppm = 1 part of
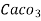 eq hardness in
eq hardness in 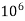 parts of water .
parts of water . - Milligram per liter is the number of milligram of
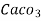 equivalent hardness present per liter of water.
equivalent hardness present per liter of water.
Thus,
1 mg/l = 1 mg of 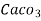 eq. hardness of 1L of water .
eq. hardness of 1L of water .
But 1 L OF Water weighs.
= 1 kg = 1000 g = 1000*1000 mg = 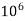 mg
mg
Therefore,
1mg/l = 1 mg of 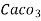 eq. per
eq. per 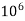 mg of water.
mg of water.
= 1 part of 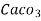 eq. per
eq. per 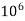 mg of water
mg of water
= 1 part of 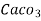 eq. per
eq. per 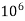 parts of water
parts of water
=1 ppm
3. Clarke’s degree is number of grains (1/7000lb) of 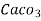 equivalent hardness per gallon (10lb) of water. Or it is parts of
equivalent hardness per gallon (10lb) of water. Or it is parts of 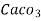 equivalent hardness per 70000 parts of water.
equivalent hardness per 70000 parts of water.
Thus,
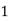 ° clark = 1 grain of
° clark = 1 grain of 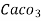 eq. hardness per gallon of water.
eq. hardness per gallon of water.
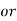 1° c l = 1 part of
1° c l = 1 part of 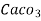 eq. hardness per 70000 parts of water .
eq. hardness per 70000 parts of water .
4. Degree French (°fr) is the part of 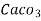 equivalent hardness per
equivalent hardness per 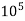 part of water .
part of water .
Thus,
1°fr = 1 part of 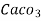 hardness eq. per
hardness eq. per 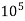 parts of water.
parts of water.
5. Milli equivalent per liter (meq / L )is the number of milli equivalents of hardness present per liter.
Thus,
1meq/L = 1 meq of 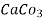 per L of water
per L of water
= 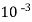 * 50 g of
* 50 g of 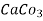 eq. per liter.
eq. per liter.
= 50 mg of 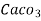 eq per liter.
eq per liter.
= 50 mg / l of 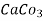 eq = 50 ppm.
eq = 50 ppm.
Relation between various units of hardness
- 1 ppm = 1 mg / L 0.1° fr = 0.07° Cl = 0.02 meq/L
- 1 mg/L = 1 ppm = 0.1° fr = 0.07° cl = 0.02 meq / L
- 1°cl = 1.433° fr = 14.3 ppm = 14.3 mg / L = 0.286 meq / L
- 1° fr = 10 ppm = 10 mg/ L = 0.7° cl = 0.2 meq / L
- 1 meq/ l = 50 mg/ l = 50ppm = 5° fr = 0.35° cl
formation)
In steam generation and to increase the life of the boiler .
For the purpose the feed water is treated well externally ( by processes like ion exchange , zoolite , soda lime ) and internally ( by processes like phosphate , conditioning , hydrazine , tretament )depending upon the operating pressure of boiler , the feed water should satisfy the following requirement.
Type of boiler | Permitted hardness of feed water |
Low pressure below 15kg/cm² | 25 – 50 ppm caco3 equivalent |
Medium pressure 15 – 30 kg/cm² | 10 – 25 ppm caco3 equivalent |
High pressure greater than 30 kg/cm² | 0 – 10 ppm caco3 equivalent |
- The most important use of water as enginnering material is the steam generation .
- Steam is required for power generation in thermal power stations for uniform and controlled heating of reactors for steam engine e.t.c .
- Different design of boilers ( water tube, vertical , e.t.c ) are used in making steam of different pressure and temprature.
- Water of minimum hardness 9 below 10 ppm is used for high pressure steam boilers ) and maximum purity ( total dissolved solids below 25 mg / lit) is good for steam generation .
- Water of higher hardness and other imparites is treated well before . fedding it in boiler.
|
Steam generation can be economized by recircuit calculating the waste steam on reheating and recircualting the steam condensalte to boiler from the steam generation.
|
Major boiler problems due to use of unsutiable water are :-
- Corrison
- Priming and foaming
- Sludges and scales formation
- Caustic embittlement
Out of these boiler troubles the corrison and seals formation are much serve and requires atmost care.
Scale and Sludge Formation:
- In boiler water evaporates continously and the concentration of salts left behind goes on increasing . After the saturation point they get precipitated.
- If the precipitate remains in boiler tube as loose and slimy matter is called sludge.
- If some of the precipitated matter adhers strongly and forms strong bad conducting layer on their inner side of boiler tube , then it is known as scale.
Scale
A] formation of scales :-
Scale is the hard and strongly adhered coating to the inner surface of boiler and it is a bad conductor of heat. It is the main source of boiler trouble.
It is caused due to :-
- Decomposition of bicarbonates :-
At high temprature bicarbonates decompose into sticky water insolube material.
Ca ( HcO3)2 ----------------- CaCO3 + H2O + CO2
Mg ( HCo3)2 - - -------------- Mg (Oh)2 + 2CO2
2. Hydrolsis of magnesium salts :-
At higher temprature magnesium salts undergo hydrolysis.
MgCl2 + 2H2O ---------------------- mg ( OH)2 + 2HCl
3. Presence of silica :-
The source of ssilica is ( form) from sand and filter .silica may be in the form of colloidal particles. And it can be deposite as calcium silicate or magnesium silicate as firmly adhering materials.
4. Decreased solublity of CaSO4 :-
CaSo4 has lesser solublity at higher temprature hence at high temprature CaSo4 present in boiler feed water will precipitate as hard scale forming materials.
|
Sludge
Formation of sludge
- The loose slimy mass of salts precipitated in boiler water is the sludge.
- They are generally formed at cooler portion of boiler and they loosely deposit in the parts of boiler tube where flow rate is slow e.g vlves bends
- Sludges are easy to remove by using brushes detergent solutions blow down opreation e.t.c .
Formula :-
Hardness of water by EDTA
1) When standard hard water is used
Total hardness of water sample = 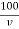 .
. 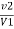 . Mg. CaCo3
. Mg. CaCo3
Where,
M = mgCaco3 in titrated standard hard water
V1 = EDTA volume for titrated standard hard water
V2 = EDTA volume for V ml water sample
2) When EDTA of known molarity used
Total hardness of water sample =  *z*
*z*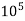 MgCo3
MgCo3
Where,
Y = EDTA volume for V ml of water sample
Z = molarity of EDTA solution
Chloride ions in water sample :-
cl- quantity = 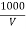 yz (35.5) mgel/L
yz (35.5) mgel/L
Where,
Y = volume of AgNo3
Z = molarity of AgNo3
Alkalinites in water sample
Phenolphthalien alkalinty
P = 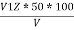 PPM Caco3 equivalent
PPM Caco3 equivalent
Methyl orange alkalinity
M = 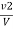 * z * 50 * 1000 ppm
* z * 50 * 1000 ppm
Where ,
V1 = volume of acid for V ml water sample in titraton with use of phenolphthalein indicator.
V2 = volume of acid for V ml water sample in the continued titration using methyl orange indicator.
Z = normally of acid in barette.
Ion exchange process:
Ion exchange technology is a proven method of producing high purity softened and demineralized water. It is used in most industries that require high purity water and to reclaim water from processes. The Ion exchange process involves the exchanging of contaminant ions for Na+ ions in a softening application and H+ and OH- ions in pure water application. Cations and anions can be removed by the cation and anion exchange resins. Resins containing –COOH, SO3H are capable for exchanging their H+ ions to cationic portion of minerals then it is called as cation exchanger while the resins containing –NH2, NHCH3 are capable for exchanging the anionic portion of the minerals then it is termed as anionic exchanger.
|
On supplying the hard water in first chamber which consists of Ca2+ or Mg2+ then the cation exchanger exchange it with H+ hence the cation exchanger absorbs the Ca2+ ions the left water are free from cations are passed to another chamber by the help of pump this water consists of anions such as Cl or SO4 on sprinkle up of these water at anion exchanger bed then it exchanged the anions and hence release the demineralise water. The absorbed cation and anion are sinked out through the outlet present in chamber.
Reverse osmosis:
Reverse osmosis is a process where the water is separated from the salts in the source water by pressure-driven transport through a membrane. This process uses semi-permeable membrane and applied pressure to preferentially induce water permeation through the membrane while rejecting salts. The RO plant uses less energy than thermal desalination process. This process uses thin-film composite membrane that too comprises of ultra thin aromatic polyamide thin film. The used polyamide film gives the transparent properties while the remaining part provides the mechanical supports. The polyamide films are very dense void free polymer with high surface area allowing for its high permeability. RO desalination plant include source water intake system, pre treatment facilities and high pressure feed pumps, RO membrane trains, energy recovery and desalinated water conditioning system. The intake system may be the open surface water intake or series of seawater beach wells. The pretreatment system may be the screening, chemical conditioning, sedimentation or filtration that totally depends on the used quality water further the filtered water is conveyed by transfer pump from filtrate water storage tank through cartridge filter and into the suction pipe of high pressure RO feed pumps. The cartridge filters are designed in such a manner that can retain 1 to 20 microns particles which remained in the source water after pretreatment. The high pressure feed pumps are designed to deliver the source water to the RO membranes at pressure required for membrane separation of the fresh water from the salts. The actual required feed pressure is site-specific and is mainly determined by the source water salinity and the configuration of the RO system.