UNIT III
ADVANCED MATERIALS
3.1.1 Introduction
Polymer chemists study large, complex molecules that are built up from many smaller units. They study about the smaller building blocks combine, and create useful materials with specific characteristics by manipulating the molecular structure of the monomers/polymers used, the composition of the monomer/polymer combinations, and applying chemical and processing techniques that can to a large extent, affect the properties of the final product. Polymer chemists are unique within the chemistry community because their understanding of the relationship between structure and property spans from the molecular scale to the macroscopic scale.
It is a long molecule formed by joining together of thousands of small molecular units by chemical bonds. Polymers are giant molecules also called macromolecules that are essential to our existence. They are important chemicals in our bodies in plants (starch, cellulose) and in our everyday lives (fibers, plastics, elastomers). Polymers are made by transforming small molecules into molecules with very large molecular weights. Although the chemical properties of polymers are similar to those of analogous small molecules, their physical properties are quite different. Every polymer has its own characteristics, but most polymers have the following general properties:
Polymers can be very resistant to chemicals.
Polymers can be both thermal and electrical insulators.
Generally, polymers are light in weight with varying degrees of strength.
Polymers can be processed in various ways to produce thin fibers or intricate parts.
Polymer is a molecule formed by joining of thousands of smaller molecular units together by chemical bonds. A chemical process that leads to the formation of polymer is known as polymerization. Degree of polymerization: The number of repeat units monomeric units available in the polymer is known as degree of polymerization.
Functionality: The number of bonding sites or reactive sites or functional groups present in the molecule. Ex: The double bond in vinyl monomers (CH2 = CHX) can be considered as a site for two free valencies. When the double bond is broken, two single bonds become available for combination.
H2C=CHX → CH2 – CHX
1. When the functionality of monomer is two bifunctional linear (or) straight chain polymer is formed. Ex: (a)vinyl monomers (b)adipic acid (c)hexamethylene diamine (d)terephthalic acid(e)ethylene glycol (f )amino acid Example for polymer: HDPE (high density polythene)
2. When the functionality of monomer is three (tri-functional), three dimensional net work polymer is formed. Ex: phenol, glycerol Examples for polymers : Urea formaldehyde, phenol formaldehyde.
3.1.2 Plastics
Synthetic or semi-synthetic polymers are plastics. Plastics used for industrial work come from petrochemicals. Plastic refers to its ability to deform without breaking. The polymer used in making plastics is usually a combination of additives, colorants, plasticizers, stabilizers, fillers, and reinforcements. These additives affect the chemical composition, properties, and mechanical properties of plastics and affect its cost.
3.1.3 Thermos softening and thermosetting plastics
Thermoplastic polymer: A thermoplastic is a resin that is solid at room temperature but becomes plastic and soft upon heating, flowing due to crystal melting or by virtue of crossing the glass transition temperature (Tg). Upon processing, usually via injection-moulding or blow-moulding-like processes, thermoplastics take the shape of the mould within which they are poured as melt, and cool to solidify into the desired shape. The significant aspect of thermoplastics is their reversibility, the ability to undergo reheating, melt again, and change shape. This allows for additional processing of the same material, even after being prepared as a solid. Processes such as extrusion, thermoforming, and injection moulding rely on such resin behaviour. Some common thermoplastic materials include polyethylene (PE), polycarbonate (PC), and polyvinyl chloride (PVC).
Thermosetting Polymer: A thermosetting resin, or thermosetting polymer, is generally a liquid material at room temperature which hardens irreversibly upon heating or chemical addition. When it is placed in a mould and heated, the most solidifies into the specified shape, but this solidification process includes the formation of certain bonds, called cross links, that hold the molecules in place and change the basic nature of the material, preventing it from melting. As a result, a there most, as opposed to a thermoplastic, cannot return to its initial phase, rendering the process irreversible. There most, upon heating, become set, fixed in a specific form. During overheating, there most tend to degrade without entering a fluid phase. Processes such as compression molding, resin transfer molding, pultrusion, hand lay-up, and filament winding depend on thermosetting polymer behavior. Some common there most include epoxy, polyimide, and phenolic, many of which are significant in composites.
3.1.4 Industrially important plastics like phenol formaldehyde
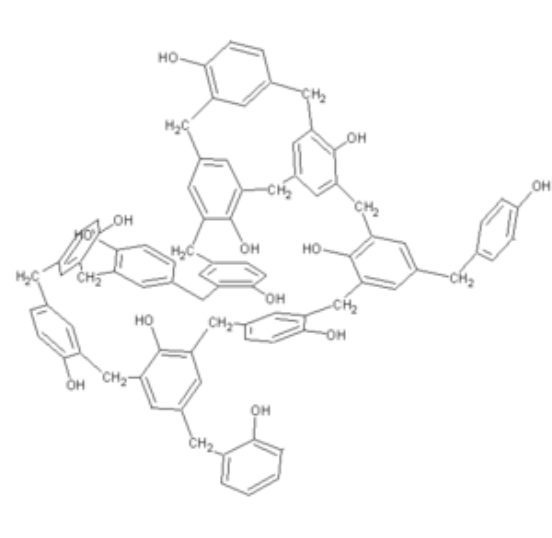
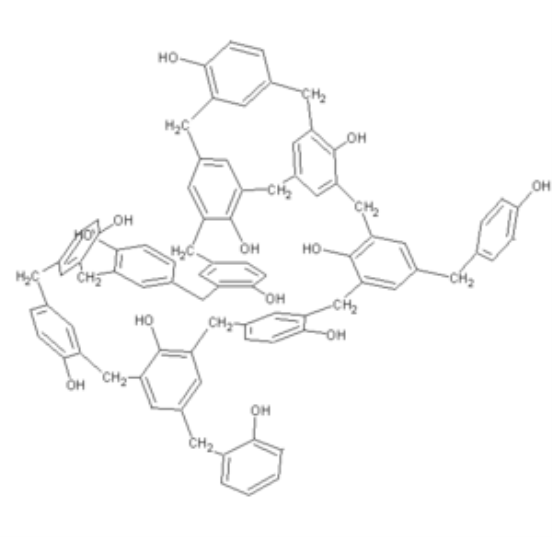
Phenol formaldehyde is the synthetic high polymers. They are formed by the reaction of phenol and substitute of phenol with formaldehyde. Besides polyurethanes and polyesters, phenolic and epoxy resins are the most prominent applications for technical lignin’s in thermo setting materials. They are formed by the step growth polymerization reaction.
|
Applications:
1) Phenolic resins are mainly used in the circuit board production that is for making circuit board like PCB.
2) It is also used in Laminated Material like Laminated sheets, rods and tubes.
3) Phenolic resin is used as a binder in loudspeaker driver suspension components which are made of cloth.
4) Phenolic resins are used in making of Duroplast, used in the Trabant automobiles.
3.1.5 Urea formaldehyde and epoxy resins
Urea formaldehyde:
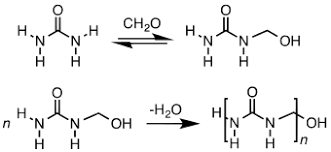
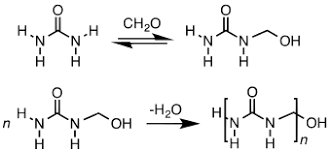
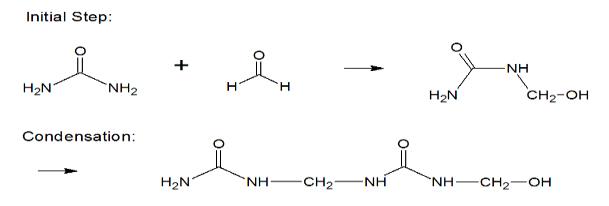
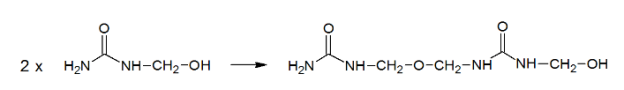
Urea-formaldehyde is also known as urea-methanal. It is a non-transparent thermosetting resin which is made from urea and formaldehyde when they are heated in the presence of a mild base such as ammonia or pyridine. These resins are commonly used in adhesives, finishes and molded objects.
A class of synthetic resins called Urea-formaldehyde resin is obtained by chemical combination of urea (a solid crystal obtained from ammonia) and formaldehyde (a highly reactive gas obtained from methane). Urea-formaldehyde resins are used mostly as adhesives for the bonding of plywood, particle board and other structured wood products.
|
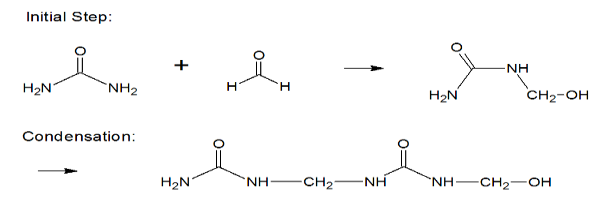
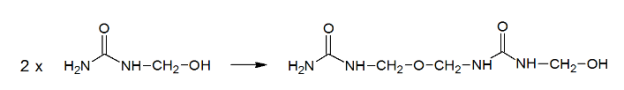
Synthesis of Urea Formaldehyde:
|
Applications:
1) It is used as an adhesive for decorative laminate applications (7%).
2) It is used in wet laid fiber glass mats, air filtration, coated and bonded abrasives.
3) Urea Formaldehyde is used as wood adhesives they are mainly used for the manufacture of wood products intended for interior use only.
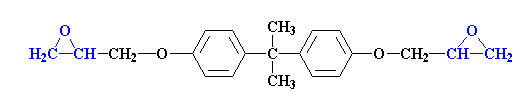
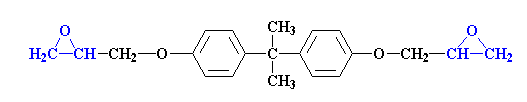
Epoxy Resins:
Epoxy resins are a wonderful chemical known for its versatile nature. Epoxy is highly useful chemical compounds; epoxy resins have carved their niche in a number of industrial applications.
|
Applications:
1) Epoxies are used in paint industry as it dries quickly and provides protective layers that are highly tough.
2) Epoxies are used as structural or engineering adhesives used in the construction of aircrafts, automobiles, boats.
3) These are an integral part of the electronic industry and used in over molding transistors, integrated circuits, PCB’s and hybrid circuits.
4) As an imperative part of aerospace industry, epoxies are used as structural matrix material.
5) In a highly technical application, epoxy resin is used for embedding samples for their use under electron microscope.
6) Not limited just to technical applications, artists have also used epoxies as a painting medium by mixing it with pigment to obtain colors.
3.1.6 Conducting polymers and Bipolymers (Introduction, examples and applications):
Conducting polymers: Conductive polymers or more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are generally not thermoplastics, i.e., they are not thermo formable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.
Applications:
1) They are anti-static substance for photographic film.
2) They are corrosion inhibitors.
3) They are compact capacitors.
4) They are anti static coated.
5) Electromagnetic shielding for computers.
6) They are used in transistors.
7) They are used in Light Emitting Diodes.
8) They are used in solar cells.
9) They are used in mobile displays, telephone and mini format television screen.
Bipolymers:
Biopolymers are polymers that are produced by living organisms. They are generally polymers of starch. These are composed of monomeric units. They can be classified based on the three main classes based on the formation of structure, length of the polymers that comprehends more than thirteen nuclei monomers or amino acids which are short lengthened amino acids.
Applications:
1) Coatings
2) Fibers
3) Plastics
4) Adhesives
5) Cosmetics
6) Oil Industry
7) Paper
8) Textiles/clothing
9) Water Treatment
10) Biomedical
11) Pharmaceutical
12) Automotive
13) Rubber
3.2.1 Introduction
Definition: -
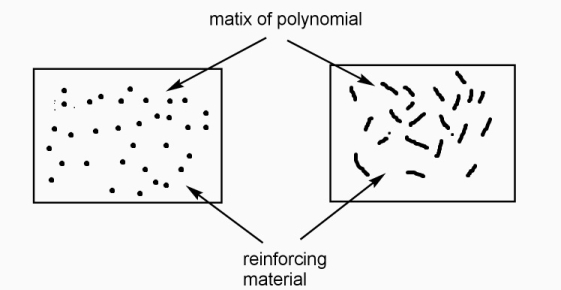
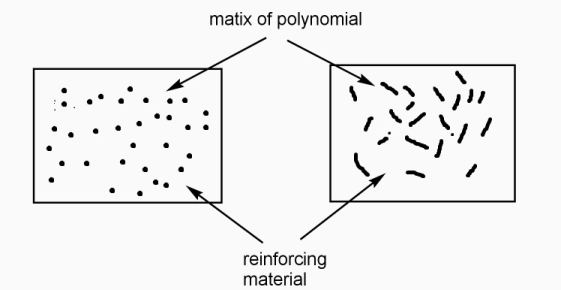
Polymer and reinforcing materials as a two-phase mixture with the interfaces between them, called as polymer composite.
Therefore,
Polymer phase called as ‘matrix phase ‘mixing constituents called as dispersed phase and boundaries between the matrix and dispersed phase, are known as interface.
|
a) Particle refinement
b) Fibre refinement
Functions of matrix constituents in polymer composite: -
- To bind the reinforcing particles / fibres strongly.
- It acts as medium for distribution of applied load to the dispersed phase.
- It keeps the reinforcing fibres in proper orientation for high strength development.
- It prevents propagation of cracks due to plasticity.
3.2.2 Composition
|
3.2.3 Properties and uses of fiber reinforced plastics (FRP) and glass reinforced plastic (GRP)
GRP is an acronym for Glass Reinforced Plastic is also often referred to as fibreglass (fibre glass in the US) or glass fibre composite and belongs to a family of products known as FRP or Fibre Reinforced Plastics. These are composed on fibres embedded in the matrix material. These can be further divided into 3 categories – continuous aligned, discontinuous aligned and discontinuous randomly oriented fibre composites.
e.g. – glass fibre reinforced composite, carbon fibre reinforced and aramid fibre reinforced composites.
Technique: -
- Resin is coated on fibre cloth or fibre mat on both the sides.
- Rollers are used to spread the resin uniformly.
- Alternative layers of the resin – fibre material arranged with same or different orientation for getting proper thickness then passing through mould for proper temperature.
Structural composites: -
(2types)
- Laminar – consists of sheets or panels with proper orientations and cemented together with a resin.
e.g. – plywood, high tensile strength.
2. Sandwiched – consists of two strong outer sheets separated by a layer of less dense material called as core.
Properties of composite: -
- Low coefficient of expansion
- Low cost of production
- High dimensional stability
- High tensile strength
- High heat stability, higher working temp.
- Better abrasion / wear resistivity.
- Better toughness and impact strength.
Applications: -
- In automobile parts, racing vehicles components.
- Parts of aircrafts.
- Boats parts.
- Sport goods, musical instruments, various toys, etc.
- High speed machinery parts, PCBS, equipment parts, bodies of refrigerator, coolers, windows and doors, etc.
UNIT III
ADVANCED MATERIALS
3.1.1 Introduction
Polymer chemists study large, complex molecules that are built up from many smaller units. They study about the smaller building blocks combine, and create useful materials with specific characteristics by manipulating the molecular structure of the monomers/polymers used, the composition of the monomer/polymer combinations, and applying chemical and processing techniques that can to a large extent, affect the properties of the final product. Polymer chemists are unique within the chemistry community because their understanding of the relationship between structure and property spans from the molecular scale to the macroscopic scale.
It is a long molecule formed by joining together of thousands of small molecular units by chemical bonds. Polymers are giant molecules also called macromolecules that are essential to our existence. They are important chemicals in our bodies in plants (starch, cellulose) and in our everyday lives (fibers, plastics, elastomers). Polymers are made by transforming small molecules into molecules with very large molecular weights. Although the chemical properties of polymers are similar to those of analogous small molecules, their physical properties are quite different. Every polymer has its own characteristics, but most polymers have the following general properties:
Polymers can be very resistant to chemicals.
Polymers can be both thermal and electrical insulators.
Generally, polymers are light in weight with varying degrees of strength.
Polymers can be processed in various ways to produce thin fibers or intricate parts.
Polymer is a molecule formed by joining of thousands of smaller molecular units together by chemical bonds. A chemical process that leads to the formation of polymer is known as polymerization. Degree of polymerization: The number of repeat units monomeric units available in the polymer is known as degree of polymerization.
Functionality: The number of bonding sites or reactive sites or functional groups present in the molecule. Ex: The double bond in vinyl monomers (CH2 = CHX) can be considered as a site for two free valencies. When the double bond is broken, two single bonds become available for combination.
H2C=CHX → CH2 – CHX
1. When the functionality of monomer is two bifunctional linear (or) straight chain polymer is formed. Ex: (a)vinyl monomers (b)adipic acid (c)hexamethylene diamine (d)terephthalic acid(e)ethylene glycol (f )amino acid Example for polymer: HDPE (high density polythene)
2. When the functionality of monomer is three (tri-functional), three dimensional net work polymer is formed. Ex: phenol, glycerol Examples for polymers : Urea formaldehyde, phenol formaldehyde.
3.1.2 Plastics
Synthetic or semi-synthetic polymers are plastics. Plastics used for industrial work come from petrochemicals. Plastic refers to its ability to deform without breaking. The polymer used in making plastics is usually a combination of additives, colorants, plasticizers, stabilizers, fillers, and reinforcements. These additives affect the chemical composition, properties, and mechanical properties of plastics and affect its cost.
3.1.3 Thermos softening and thermosetting plastics
Thermoplastic polymer: A thermoplastic is a resin that is solid at room temperature but becomes plastic and soft upon heating, flowing due to crystal melting or by virtue of crossing the glass transition temperature (Tg). Upon processing, usually via injection-moulding or blow-moulding-like processes, thermoplastics take the shape of the mould within which they are poured as melt, and cool to solidify into the desired shape. The significant aspect of thermoplastics is their reversibility, the ability to undergo reheating, melt again, and change shape. This allows for additional processing of the same material, even after being prepared as a solid. Processes such as extrusion, thermoforming, and injection moulding rely on such resin behaviour. Some common thermoplastic materials include polyethylene (PE), polycarbonate (PC), and polyvinyl chloride (PVC).
Thermosetting Polymer: A thermosetting resin, or thermosetting polymer, is generally a liquid material at room temperature which hardens irreversibly upon heating or chemical addition. When it is placed in a mould and heated, the most solidifies into the specified shape, but this solidification process includes the formation of certain bonds, called cross links, that hold the molecules in place and change the basic nature of the material, preventing it from melting. As a result, a there most, as opposed to a thermoplastic, cannot return to its initial phase, rendering the process irreversible. There most, upon heating, become set, fixed in a specific form. During overheating, there most tend to degrade without entering a fluid phase. Processes such as compression molding, resin transfer molding, pultrusion, hand lay-up, and filament winding depend on thermosetting polymer behavior. Some common there most include epoxy, polyimide, and phenolic, many of which are significant in composites.
3.1.4 Industrially important plastics like phenol formaldehyde
Phenol formaldehyde is the synthetic high polymers. They are formed by the reaction of phenol and substitute of phenol with formaldehyde. Besides polyurethanes and polyesters, phenolic and epoxy resins are the most prominent applications for technical lignin’s in thermo setting materials. They are formed by the step growth polymerization reaction.
|
Applications:
1) Phenolic resins are mainly used in the circuit board production that is for making circuit board like PCB.
2) It is also used in Laminated Material like Laminated sheets, rods and tubes.
3) Phenolic resin is used as a binder in loudspeaker driver suspension components which are made of cloth.
4) Phenolic resins are used in making of Duroplast, used in the Trabant automobiles.
3.1.5 Urea formaldehyde and epoxy resins
Urea formaldehyde:
Urea-formaldehyde is also known as urea-methanal. It is a non-transparent thermosetting resin which is made from urea and formaldehyde when they are heated in the presence of a mild base such as ammonia or pyridine. These resins are commonly used in adhesives, finishes and molded objects.
A class of synthetic resins called Urea-formaldehyde resin is obtained by chemical combination of urea (a solid crystal obtained from ammonia) and formaldehyde (a highly reactive gas obtained from methane). Urea-formaldehyde resins are used mostly as adhesives for the bonding of plywood, particle board and other structured wood products.
|
Synthesis of Urea Formaldehyde:
|
Applications:
1) It is used as an adhesive for decorative laminate applications (7%).
2) It is used in wet laid fiber glass mats, air filtration, coated and bonded abrasives.
3) Urea Formaldehyde is used as wood adhesives they are mainly used for the manufacture of wood products intended for interior use only.
Epoxy Resins:
Epoxy resins are a wonderful chemical known for its versatile nature. Epoxy is highly useful chemical compounds; epoxy resins have carved their niche in a number of industrial applications.
|
Applications:
1) Epoxies are used in paint industry as it dries quickly and provides protective layers that are highly tough.
2) Epoxies are used as structural or engineering adhesives used in the construction of aircrafts, automobiles, boats.
3) These are an integral part of the electronic industry and used in over molding transistors, integrated circuits, PCB’s and hybrid circuits.
4) As an imperative part of aerospace industry, epoxies are used as structural matrix material.
5) In a highly technical application, epoxy resin is used for embedding samples for their use under electron microscope.
6) Not limited just to technical applications, artists have also used epoxies as a painting medium by mixing it with pigment to obtain colors.
3.1.6 Conducting polymers and Bipolymers (Introduction, examples and applications):
Conducting polymers: Conductive polymers or more precisely, intrinsically conducting polymers (ICPs) are organic polymers that conduct electricity. Such compounds may have metallic conductivity or can be semiconductors. The biggest advantage of conductive polymers is their processability, mainly by dispersion. Conductive polymers are generally not thermoplastics, i.e., they are not thermo formable. But, like insulating polymers, they are organic materials. They can offer high electrical conductivity but do not show similar mechanical properties to other commercially available polymers. The electrical properties can be fine-tuned using the methods of organic synthesis and by advanced dispersion techniques.
Applications:
1) They are anti-static substance for photographic film.
2) They are corrosion inhibitors.
3) They are compact capacitors.
4) They are anti static coated.
5) Electromagnetic shielding for computers.
6) They are used in transistors.
7) They are used in Light Emitting Diodes.
8) They are used in solar cells.
9) They are used in mobile displays, telephone and mini format television screen.
Bipolymers:
Biopolymers are polymers that are produced by living organisms. They are generally polymers of starch. These are composed of monomeric units. They can be classified based on the three main classes based on the formation of structure, length of the polymers that comprehends more than thirteen nuclei monomers or amino acids which are short lengthened amino acids.
Applications:
1) Coatings
2) Fibers
3) Plastics
4) Adhesives
5) Cosmetics
6) Oil Industry
7) Paper
8) Textiles/clothing
9) Water Treatment
10) Biomedical
11) Pharmaceutical
12) Automotive
13) Rubber
3.2.1 Introduction
Definition: -
Polymer and reinforcing materials as a two-phase mixture with the interfaces between them, called as polymer composite.
Therefore,
Polymer phase called as ‘matrix phase ‘mixing constituents called as dispersed phase and boundaries between the matrix and dispersed phase, are known as interface.
|
a) Particle refinement
b) Fibre refinement
Functions of matrix constituents in polymer composite: -
- To bind the reinforcing particles / fibres strongly.
- It acts as medium for distribution of applied load to the dispersed phase.
- It keeps the reinforcing fibres in proper orientation for high strength development.
- It prevents propagation of cracks due to plasticity.
3.2.2 Composition
|
3.2.3 Properties and uses of fiber reinforced plastics (FRP) and glass reinforced plastic (GRP)
GRP is an acronym for Glass Reinforced Plastic is also often referred to as fibreglass (fibre glass in the US) or glass fibre composite and belongs to a family of products known as FRP or Fibre Reinforced Plastics. These are composed on fibres embedded in the matrix material. These can be further divided into 3 categories – continuous aligned, discontinuous aligned and discontinuous randomly oriented fibre composites.
e.g. – glass fibre reinforced composite, carbon fibre reinforced and aramid fibre reinforced composites.
Technique: -
- Resin is coated on fibre cloth or fibre mat on both the sides.
- Rollers are used to spread the resin uniformly.
- Alternative layers of the resin – fibre material arranged with same or different orientation for getting proper thickness then passing through mould for proper temperature.
Structural composites: -
(2types)
- Laminar – consists of sheets or panels with proper orientations and cemented together with a resin.
e.g. – plywood, high tensile strength.
2. Sandwiched – consists of two strong outer sheets separated by a layer of less dense material called as core.
Properties of composite: -
- Low coefficient of expansion
- Low cost of production
- High dimensional stability
- High tensile strength
- High heat stability, higher working temp.
- Better abrasion / wear resistivity.
- Better toughness and impact strength.
Applications: -
- In automobile parts, racing vehicles components.
- Parts of aircrafts.
- Boats parts.
- Sport goods, musical instruments, various toys, etc.
- High speed machinery parts, PCBS, equipment parts, bodies of refrigerator, coolers, windows and doors, etc.