UNIT V
CORROSION
Corrosion is the disintegration of a metal due to the chemical reactions between the metal and the surrounding environment. Both the types of metal and the environmental conditions, particularly gasses that come in contact with the metal, determine the form and rate of the metal.
All metals can corrode. Some metals, like pure iron, deteriorate very fast. Stainless steel, and, metals that combines with iron and other alloys, is slower to corrode and is therefore used more efficiently.
All small group of metals, are called the Noble Metals, and show much less reaction than others. As a result, they deteriorate rarely. They are, in fact, the only metals that are found in nature in their purest form. The Noble Metals, not unexpectedly, are often very valuable. They include gold, rhodium, silver, palladium, and platinum.
Causes of Corrosion:
Corrosion is the electrochemical process that occurs in various forms such as chemical forms and atmospheric forms. On the contact of acid sucstance with iron it pretend to form rust. Rust is the result of corroding steel after the iron (Fe) particles have been exposed to oxygen and moisture. When steel is exposed to water, the iron particles are lost to the water’s acidic electrolytes. The iron particles then become oxidized, which results in the formation of Fe⁺⁺. When Fe⁺⁺ is formed, two electrons are released and flow through the steel to another area of the steel known as the cathodic area.
Oxygen causes these electrons to rise up and form hydroxyl ions (OH). The hydroxyl ions react with the Fe2+ to form hydrous iron oxide, better known as rust. Where the affected iron particles were, has now become a corrosion pit, and where they are now, is called the corrosion product (rust).
There are several types of corrosion:
(i) Galvanic Corrosion: Galvanic corrosion is the most common corrosion which can be get in notice. This corrosion occurs when two different type of metals are in contact with each other in the presence of electrolyte. In this type of corrosion noble metal are safe while the active metals corrodes.
(ii) Pitting Corrosion: This type of corrosion occurs at certain conditions, there is a accelerated corrosion in some areas rather than the uniform corrosion over the substance. This condition includes low level of concentration of oxygen or high concentration of chlorides.
(iii) Microbial Corrosion: Microbial Corrosion is caused by micro-organisms. They commonly referred to as microbiologically influenced corrosion. It applies to both metallic and non-metallic materials with or without oxygen. In the presence of oxygen there are some bacteria that directly oxidize iron to iron oxides and hydroxides while in the absence of oxygen sulphate reducing bacteria are active and produce hydrogen sulphide causes sulphide stress cracking.
(iv) High Temperature Corrosion: The deterioration caused on the metal due to heating. This can cause when the metal is kept in hot atmosphere that too in the presence of oxygen, sulphur, or with any other compound which is capable of oxidizing the material.
(v) Crevice Corrosion: This occurs in confined spaces where access of fluid from the environment is limited such as gaps and contact areas between parts, under gaskets or seals, inside cracks and seams and spaces filled with deposits.
The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust. This reaction is not instantaneous; it generally proceeds over a considerably large time frame. The oxygen atoms bond with iron atoms, resulting in the formation of iron oxides. This weakens the bonds between the iron atoms in the object/structure.
The reaction of the rusting of iron involves an increase in the oxidation state of iron, accompanied by a loss of electrons. Rust is mostly made up of two different oxides of iron that vary in the oxidation state of the iron atom. These oxides are:
- Iron(II) oxide or ferrous oxide. The oxidation state of iron in this compound is +2 and its chemical formula is FeO.
- Iron(III) oxide or ferric oxide, where the iron atom exhibits an oxidation state of +3. The chemical formula of this compound is Fe2O3.
Oxygen is a very good oxidizing agent whereas iron is a reducing agent. Therefore, the iron atom readily gives up electrons when exposed to oxygen. The chemical reaction is given by:
Fe → Fe2+ + 2e–
The oxidation state of iron is further increased by the oxygen atom when water is present.
4Fe2+ + O2 → 4Fe3+ + 2O2-
Now, the following acid-base reactions occur between the iron cations and the water molecules.
Fe2+ + 2H2O ⇌ Fe(OH)2 + 2H+
Fe3+ + 3H2O ⇌ Fe(OH)3 + 3H+
The hydroxides of iron are also formed from the direct reaction between the iron cations and hydroxide ions.
O2 + H2O + 4e– → 4OH–
Fe2+ + 2OH– → Fe(OH)2
Fe3+ + 3OH– → Fe(OH)3
The resulting hydroxides of iron now undergo dehydration to yield the iron oxides that constitute rust. This process involves many chemical reactions, some of which are listed below.
- Fe(OH)2 ⇌ FeO + H2O
- 4Fe(OH)2 + O2 + xH2O → 2Fe2O3.(x+4)H2O
- Fe(OH)3 ⇌ FeO(OH) + H2O
- FeO(OH) ⇌ Fe2O3 + H2O
One similarity between all the chemical reactions listed above is that all of them are dependent on the presence of water and oxygen. Therefore, the rusting of iron can be controlled by limiting the amount of oxygen and water surrounding the metal.
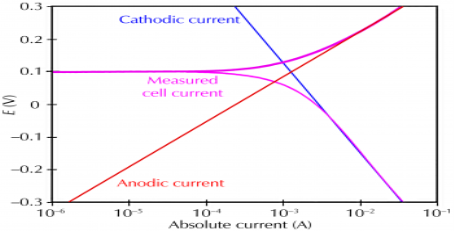
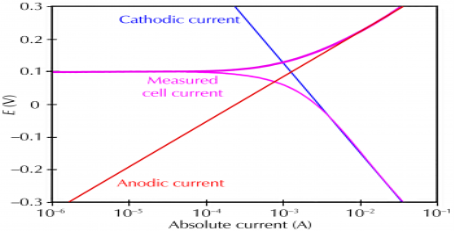
Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by the elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
|
A] Nature of metal
B] Nature of environment
Nature of metal:
Position of metal in galvanic series: -
- When 2 metals are in the contact in the presence of the electrolyte then more active metal undergo corrosion.
- Greater the difference in their positions, higher is the rate of corrosion.
Relative areas of anode and cathode: -
- If the anode < cathode then rate of corrosion.
- If the cathode < anode
Purity of metal: -
- Pure metal resists the corrosion
- Rate of corrosion increases with increases in of impurities which from galvanic cells.
Physical state: -
- Smaller the grain size of metal alloy greater the mate of corrosion.
e.g: - steel get corroded fast than the cost iron because
grain size of steel < grain size of cast ion.
Nature of oxide film: -
- If oxide layer – porous
Then corrosion take place
- If oxide layer – nonporous
Then no corrosion takes place
Over – vtg (over – potential): -
- To restore position of metal some extra VTG is required is called as over –vtg
- Higher the over vtg less is the rate of corrosion.
e.g: -Zn,Pb,Cr,Ni, etc.
B] Nature of environment:
1) Temperature:
- Higher is temperature higher is the rate of dry corrosion because attacking gas and metal get activated @ higher temp.
- Higher is the temperature higher is rate of wet corrosion because: -
High tempo, according to nearest equation electrode potential is high.
2) Moisture:
- Higher is the moisture
Higher is the rate of dry and wet corrosion
3) PH:
- Generally acidic media have more corrosive effect on metals.
4) Conductivity of medium:
- High the electrical conductivity of aq. conducting medium higher is the rate of corrosion.
5) Nature of ions:
- Cl -, no3 – have the ability break nonporous oxide layer and cause wet corrosion.
- Presence of oxalate, m phosphate and silicate ions have the ability to slow down rate of wet corrosion.
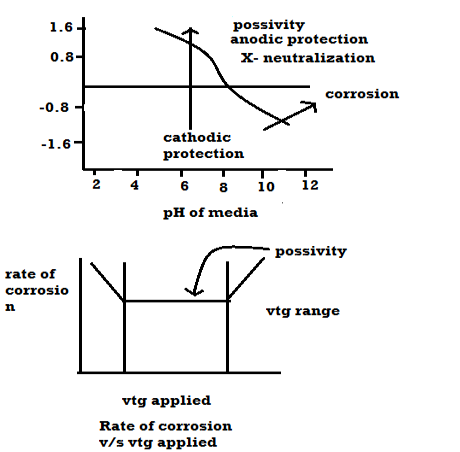
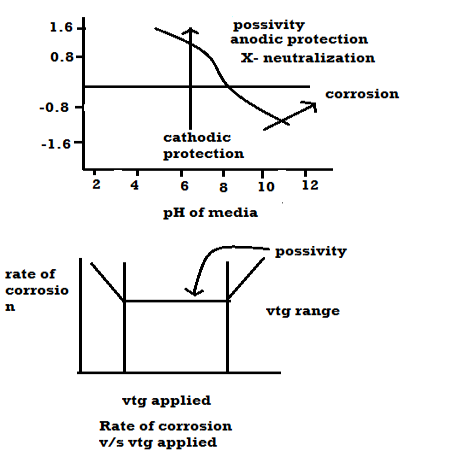
Pour baix diagrams
Pour baix diagrams are the plots of:
A] Potentials of metal VS PH of media
B] Rate of corrosion vs voltage applied
|
- Plot ai. e potentials v/j ph. it shows corrosion zone passivity zone and optimum PH at which metal has mi corrosion.
- Plot b rate of corrosion v/j vet it shows vtg range over which the metal is passive and resist the action of medium.
Merits: -
- It is used to study corrosion phenomenon.
- It also gives an into about how the corrosion is expected and can be minimized.
- It is used to do study protection of metal from the corrosion.
Demerits: -
- These diagrams are applicable to pure substances only.
- These diagrams are derived for selected conc. of ionic species.
Alteration of the environment
Changing Mediums: Change in the environment provides a versatile means for reducing corrosion. Typical changes in the medium that are often employed are lowering temperature, decreasing velocity, removing oxygen or oxidizers, and changing concentration.
Lowering temperature: This usually causes a pronounced decrease in corrosion rate. However, under some conditions, temperature changes have little effect on corrosion rate. In other cases, increasing temperature decreases attack. This phenomenon occurs as hot, fresh or salt water is raised to the boiling point and is the result of the decrease in oxygen solubility with temperature. Boiling sea water is therefore less corrosive than hot sea water.
Decreasing velocity: Velocity generally increases corrosive attack, although there are some important exceptions. Metals and alloys that passivate, such as stainless steels, generally have better resistance to flowing mediums than stagnant solutions. Very high velocities should be always avoided where possible, because of erosion-corrosion effects.
Changing concentration: Decreasing corrosive concentration is usually elective. In many processes, the presence of a corrosive is accidental. E.g.: corrosion by the water coolant in nuclear reactors is reduced by eliminating chloride ions. Many acids such as sulphuric and phosphoric are virtually inert at high concentrations at moderate temperatures.
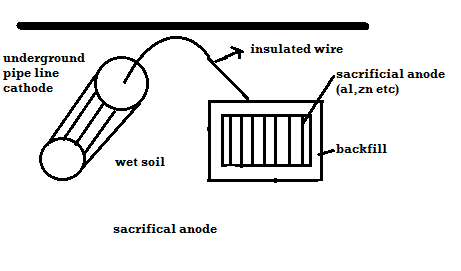
Principle:
The metal to be protected is forced to behave as cathode.
Two types:
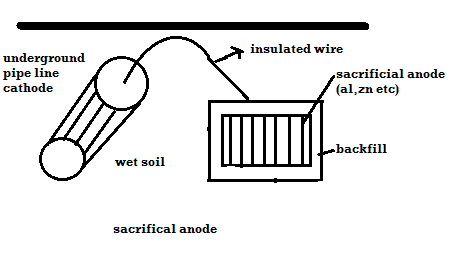
A) By using sacrificial anode
Methods / processes:
- The metallic structure to be protected from corrosion is connected to the anodic metal by insulated wire.
- Anodic metal is more reactive like Zn etc.
- Anodic metal corrosion itself but it protects the cathodic metal from corrosion .it is called as sacrificial metal.
- Anodic metal is kept in back fill (mixture of coal and Nacl) to increase electrical contact with surrounding soil.
- When anodic metal consumed completely then it is replaced with fresh piece of metal
|
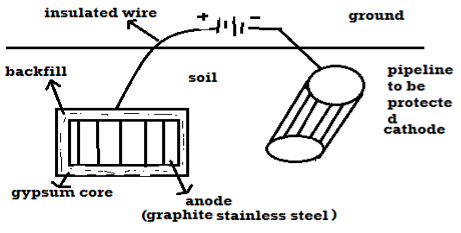
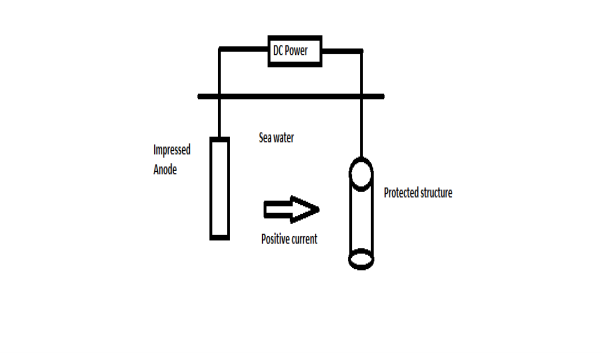
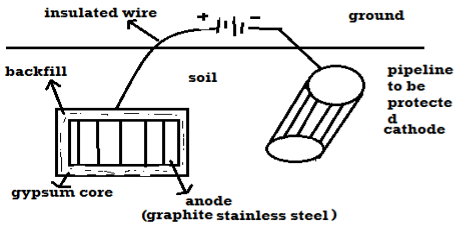
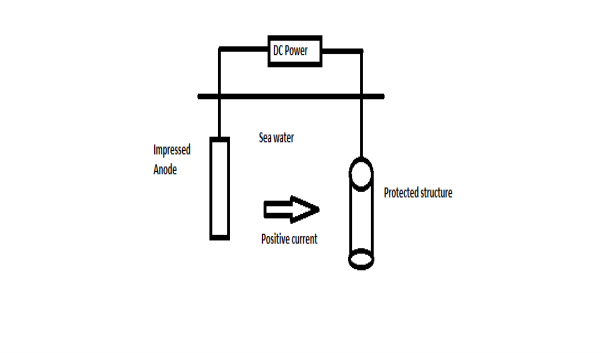
B) By using impressed current: -
Method: -
- In this method an impressed current is applied in opposite to nullify the corrosion current and convert the corroding metal from anode to the cathode.
- The impressed current Is derived from DC source and given to insoluble anode like graphite, stainless steel which buried in soil.
- The –ve terminal of DC source is connected to pipeline to be protected from corrosion.
- Anodic metal is kept in black fill to increase electrical contact with the surrounding soil.
|
Limitations:
- Highly investment and maintenance cost
- Special case needs to be taken to see the structure is not over protected otherwise H2 liberation maybe occur.
Hot dipping processes:
|
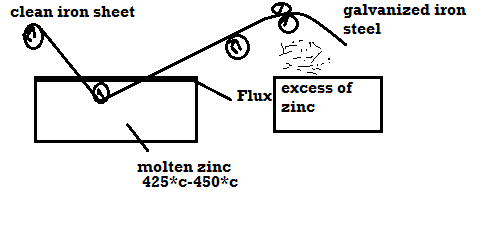
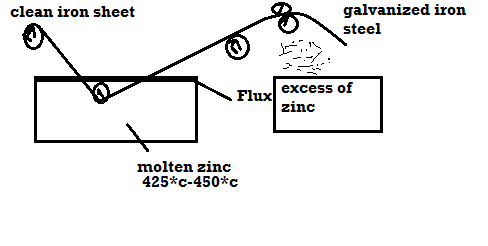
1) Galvanizing:
Method:
- Coating of Zn on the steel or iron is called as galvanizing.
- Steel article is cleaned well with dil H2SO4 then with water and dry it.
- Then the steel article is dipped in molten Zn bath and it is maintained at the temp 425 – 450 c.
- The molten Zn bath is covered with flux.
- Then the article is allowed to pass through to mate uniform coating layer and to remove excesses of Zn.
|
Applications: -
- Various galvanized iron (G.I) articles are used.
- It is useful for the protection of iron.
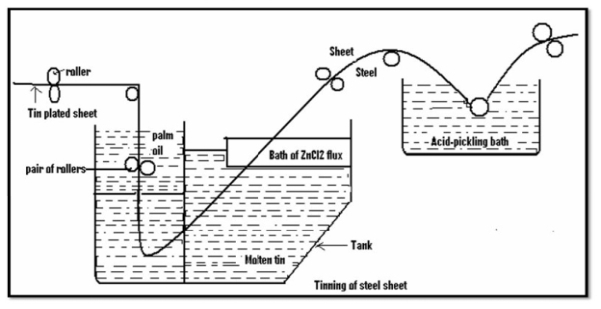
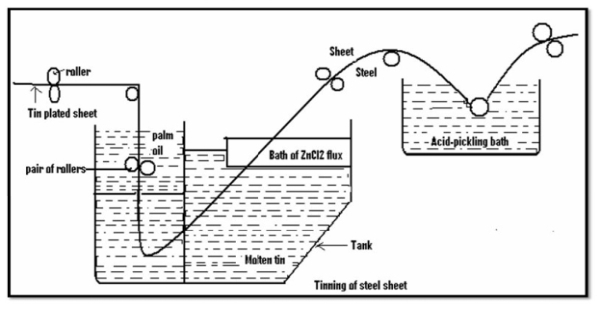
Tinning: -
Method: -
- Coating of tin on a base metal i.e steel iron is called as tinning.
- The steel article is cleaned well and then passes through molten tin bath which is maintained at temp 240c.
- Then, the article is allowed to pass through palm oil bath which protects the hot tin coated surface against corrosion.
- Then the article is allowed to pass through to make uniform coating layer to remove excess of tin.
|
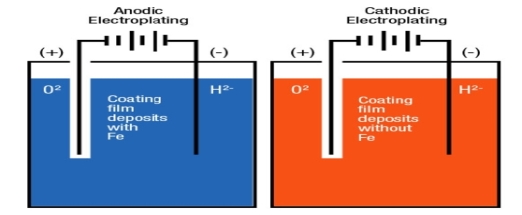
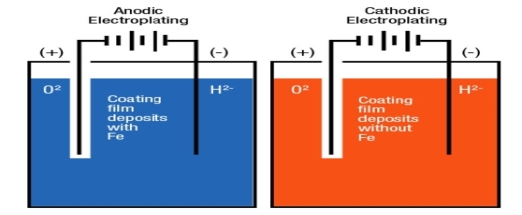
Principle:
Electroplating is a method in which coating metal is coated on the base metal on the basis ofelectrolysis principle.
Processes:
- The article to be electroplated is cleaned well.
- There is a non-conducting tank which containing coating metal salts.
- The article is connected to the negative terminal of DC source which act as a cathode.
- Anode is a coating metal.
- After adjusting suitable PH and density on the base metal, then this method is started.
|
Metal ions in a solution migrate towards the article and get deposit on the base metal in the form of coating layer.
- Cr + 3e- - Cr (if cr plating)
- Ag+ + Ag - Ag (if Ag plating)
- Ni + + 2e- - Ni (if Ni plating)
At anode (Coatingmetal)
- Cr - Cr + + 3e –
- Ag - Ag + + e-
- Ni - Ni + + 2e-
Advantages: -
- Electroplating can be done on the article of any shape
- This is strong coating
Application: -
- Corrosion protection method
- Decoration
- It is also applicable on the non – metallic surface
- Electroplating is done on the many parts of machines.
- Coating metals: -Cu,Ni,Cr, Ag, etc.
- Base metals: - Fe (steel)non-metallic surface like glass.
UNIT V
CORROSION
Corrosion is the disintegration of a metal due to the chemical reactions between the metal and the surrounding environment. Both the types of metal and the environmental conditions, particularly gasses that come in contact with the metal, determine the form and rate of the metal.
All metals can corrode. Some metals, like pure iron, deteriorate very fast. Stainless steel, and, metals that combines with iron and other alloys, is slower to corrode and is therefore used more efficiently.
All small group of metals, are called the Noble Metals, and show much less reaction than others. As a result, they deteriorate rarely. They are, in fact, the only metals that are found in nature in their purest form. The Noble Metals, not unexpectedly, are often very valuable. They include gold, rhodium, silver, palladium, and platinum.
Causes of Corrosion:
Corrosion is the electrochemical process that occurs in various forms such as chemical forms and atmospheric forms. On the contact of acid sucstance with iron it pretend to form rust. Rust is the result of corroding steel after the iron (Fe) particles have been exposed to oxygen and moisture. When steel is exposed to water, the iron particles are lost to the water’s acidic electrolytes. The iron particles then become oxidized, which results in the formation of Fe⁺⁺. When Fe⁺⁺ is formed, two electrons are released and flow through the steel to another area of the steel known as the cathodic area.
Oxygen causes these electrons to rise up and form hydroxyl ions (OH). The hydroxyl ions react with the Fe2+ to form hydrous iron oxide, better known as rust. Where the affected iron particles were, has now become a corrosion pit, and where they are now, is called the corrosion product (rust).
There are several types of corrosion:
(i) Galvanic Corrosion: Galvanic corrosion is the most common corrosion which can be get in notice. This corrosion occurs when two different type of metals are in contact with each other in the presence of electrolyte. In this type of corrosion noble metal are safe while the active metals corrodes.
(ii) Pitting Corrosion: This type of corrosion occurs at certain conditions, there is a accelerated corrosion in some areas rather than the uniform corrosion over the substance. This condition includes low level of concentration of oxygen or high concentration of chlorides.
(iii) Microbial Corrosion: Microbial Corrosion is caused by micro-organisms. They commonly referred to as microbiologically influenced corrosion. It applies to both metallic and non-metallic materials with or without oxygen. In the presence of oxygen there are some bacteria that directly oxidize iron to iron oxides and hydroxides while in the absence of oxygen sulphate reducing bacteria are active and produce hydrogen sulphide causes sulphide stress cracking.
(iv) High Temperature Corrosion: The deterioration caused on the metal due to heating. This can cause when the metal is kept in hot atmosphere that too in the presence of oxygen, sulphur, or with any other compound which is capable of oxidizing the material.
(v) Crevice Corrosion: This occurs in confined spaces where access of fluid from the environment is limited such as gaps and contact areas between parts, under gaskets or seals, inside cracks and seams and spaces filled with deposits.
The exposure of iron (or an alloy of iron) to oxygen in the presence of moisture leads to the formation of rust. This reaction is not instantaneous; it generally proceeds over a considerably large time frame. The oxygen atoms bond with iron atoms, resulting in the formation of iron oxides. This weakens the bonds between the iron atoms in the object/structure.
The reaction of the rusting of iron involves an increase in the oxidation state of iron, accompanied by a loss of electrons. Rust is mostly made up of two different oxides of iron that vary in the oxidation state of the iron atom. These oxides are:
- Iron(II) oxide or ferrous oxide. The oxidation state of iron in this compound is +2 and its chemical formula is FeO.
- Iron(III) oxide or ferric oxide, where the iron atom exhibits an oxidation state of +3. The chemical formula of this compound is Fe2O3.
Oxygen is a very good oxidizing agent whereas iron is a reducing agent. Therefore, the iron atom readily gives up electrons when exposed to oxygen. The chemical reaction is given by:
Fe → Fe2+ + 2e–
The oxidation state of iron is further increased by the oxygen atom when water is present.
4Fe2+ + O2 → 4Fe3+ + 2O2-
Now, the following acid-base reactions occur between the iron cations and the water molecules.
Fe2+ + 2H2O ⇌ Fe(OH)2 + 2H+
Fe3+ + 3H2O ⇌ Fe(OH)3 + 3H+
The hydroxides of iron are also formed from the direct reaction between the iron cations and hydroxide ions.
O2 + H2O + 4e– → 4OH–
Fe2+ + 2OH– → Fe(OH)2
Fe3+ + 3OH– → Fe(OH)3
The resulting hydroxides of iron now undergo dehydration to yield the iron oxides that constitute rust. This process involves many chemical reactions, some of which are listed below.
- Fe(OH)2 ⇌ FeO + H2O
- 4Fe(OH)2 + O2 + xH2O → 2Fe2O3.(x+4)H2O
- Fe(OH)3 ⇌ FeO(OH) + H2O
- FeO(OH) ⇌ Fe2O3 + H2O
One similarity between all the chemical reactions listed above is that all of them are dependent on the presence of water and oxygen. Therefore, the rusting of iron can be controlled by limiting the amount of oxygen and water surrounding the metal.
Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by the elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
|
A] Nature of metal
B] Nature of environment
Nature of metal:
Position of metal in galvanic series: -
- When 2 metals are in the contact in the presence of the electrolyte then more active metal undergo corrosion.
- Greater the difference in their positions, higher is the rate of corrosion.
Relative areas of anode and cathode: -
- If the anode < cathode then rate of corrosion.
- If the cathode < anode
Purity of metal: -
- Pure metal resists the corrosion
- Rate of corrosion increases with increases in of impurities which from galvanic cells.
Physical state: -
- Smaller the grain size of metal alloy greater the mate of corrosion.
e.g: - steel get corroded fast than the cost iron because
grain size of steel < grain size of cast ion.
Nature of oxide film: -
- If oxide layer – porous
Then corrosion take place
- If oxide layer – nonporous
Then no corrosion takes place
Over – vtg (over – potential): -
- To restore position of metal some extra VTG is required is called as over –vtg
- Higher the over vtg less is the rate of corrosion.
e.g: -Zn,Pb,Cr,Ni, etc.
B] Nature of environment:
1) Temperature:
- Higher is temperature higher is the rate of dry corrosion because attacking gas and metal get activated @ higher temp.
- Higher is the temperature higher is rate of wet corrosion because: -
High tempo, according to nearest equation electrode potential is high.
2) Moisture:
- Higher is the moisture
Higher is the rate of dry and wet corrosion
3) PH:
- Generally acidic media have more corrosive effect on metals.
4) Conductivity of medium:
- High the electrical conductivity of aq. conducting medium higher is the rate of corrosion.
5) Nature of ions:
- Cl -, no3 – have the ability break nonporous oxide layer and cause wet corrosion.
- Presence of oxalate, m phosphate and silicate ions have the ability to slow down rate of wet corrosion.
Pour baix diagrams
Pour baix diagrams are the plots of:
A] Potentials of metal VS PH of media
B] Rate of corrosion vs voltage applied
|
- Plot ai. e potentials v/j ph. it shows corrosion zone passivity zone and optimum PH at which metal has mi corrosion.
- Plot b rate of corrosion v/j vet it shows vtg range over which the metal is passive and resist the action of medium.
Merits: -
- It is used to study corrosion phenomenon.
- It also gives an into about how the corrosion is expected and can be minimized.
- It is used to do study protection of metal from the corrosion.
Demerits: -
- These diagrams are applicable to pure substances only.
- These diagrams are derived for selected conc. of ionic species.
Alteration of the environment
Changing Mediums: Change in the environment provides a versatile means for reducing corrosion. Typical changes in the medium that are often employed are lowering temperature, decreasing velocity, removing oxygen or oxidizers, and changing concentration.
Lowering temperature: This usually causes a pronounced decrease in corrosion rate. However, under some conditions, temperature changes have little effect on corrosion rate. In other cases, increasing temperature decreases attack. This phenomenon occurs as hot, fresh or salt water is raised to the boiling point and is the result of the decrease in oxygen solubility with temperature. Boiling sea water is therefore less corrosive than hot sea water.
Decreasing velocity: Velocity generally increases corrosive attack, although there are some important exceptions. Metals and alloys that passivate, such as stainless steels, generally have better resistance to flowing mediums than stagnant solutions. Very high velocities should be always avoided where possible, because of erosion-corrosion effects.
Changing concentration: Decreasing corrosive concentration is usually elective. In many processes, the presence of a corrosive is accidental. E.g.: corrosion by the water coolant in nuclear reactors is reduced by eliminating chloride ions. Many acids such as sulphuric and phosphoric are virtually inert at high concentrations at moderate temperatures.
Principle:
The metal to be protected is forced to behave as cathode.
Two types:
A) By using sacrificial anode
Methods / processes:
- The metallic structure to be protected from corrosion is connected to the anodic metal by insulated wire.
- Anodic metal is more reactive like Zn etc.
- Anodic metal corrosion itself but it protects the cathodic metal from corrosion .it is called as sacrificial metal.
- Anodic metal is kept in back fill (mixture of coal and Nacl) to increase electrical contact with surrounding soil.
- When anodic metal consumed completely then it is replaced with fresh piece of metal
|
B) By using impressed current: -
Method: -
- In this method an impressed current is applied in opposite to nullify the corrosion current and convert the corroding metal from anode to the cathode.
- The impressed current Is derived from DC source and given to insoluble anode like graphite, stainless steel which buried in soil.
- The –ve terminal of DC source is connected to pipeline to be protected from corrosion.
- Anodic metal is kept in black fill to increase electrical contact with the surrounding soil.
|
Limitations:
- Highly investment and maintenance cost
- Special case needs to be taken to see the structure is not over protected otherwise H2 liberation maybe occur.
Hot dipping processes:
|
1) Galvanizing:
Method:
- Coating of Zn on the steel or iron is called as galvanizing.
- Steel article is cleaned well with dil H2SO4 then with water and dry it.
- Then the steel article is dipped in molten Zn bath and it is maintained at the temp 425 – 450 c.
- The molten Zn bath is covered with flux.
- Then the article is allowed to pass through to mate uniform coating layer and to remove excesses of Zn.
|
Applications: -
- Various galvanized iron (G.I) articles are used.
- It is useful for the protection of iron.
Tinning: -
Method: -
- Coating of tin on a base metal i.e steel iron is called as tinning.
- The steel article is cleaned well and then passes through molten tin bath which is maintained at temp 240c.
- Then, the article is allowed to pass through palm oil bath which protects the hot tin coated surface against corrosion.
- Then the article is allowed to pass through to make uniform coating layer to remove excess of tin.
|
Principle:
Electroplating is a method in which coating metal is coated on the base metal on the basis ofelectrolysis principle.
Processes:
- The article to be electroplated is cleaned well.
- There is a non-conducting tank which containing coating metal salts.
- The article is connected to the negative terminal of DC source which act as a cathode.
- Anode is a coating metal.
- After adjusting suitable PH and density on the base metal, then this method is started.
|
Metal ions in a solution migrate towards the article and get deposit on the base metal in the form of coating layer.
- Cr + 3e- - Cr (if cr plating)
- Ag+ + Ag - Ag (if Ag plating)
- Ni + + 2e- - Ni (if Ni plating)
At anode (Coatingmetal)
- Cr - Cr + + 3e –
- Ag - Ag + + e-
- Ni - Ni + + 2e-
Advantages: -
- Electroplating can be done on the article of any shape
- This is strong coating
Application: -
- Corrosion protection method
- Decoration
- It is also applicable on the non – metallic surface
- Electroplating is done on the many parts of machines.
- Coating metals: -Cu,Ni,Cr, Ag, etc.
- Base metals: - Fe (steel)non-metallic surface like glass.