UNIT 6
Metallic materials and Green Chemistry
6.1.1 Introduction
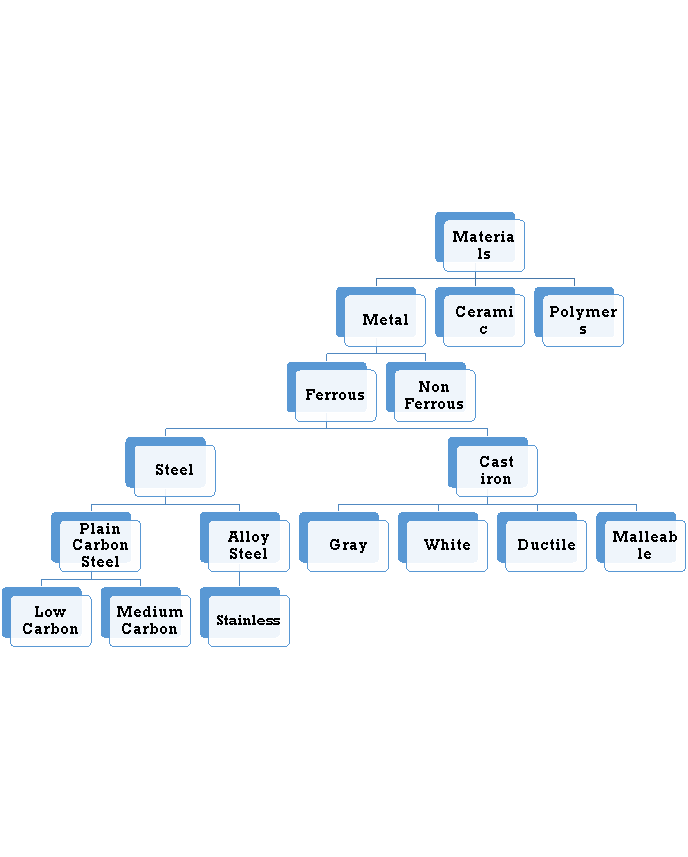
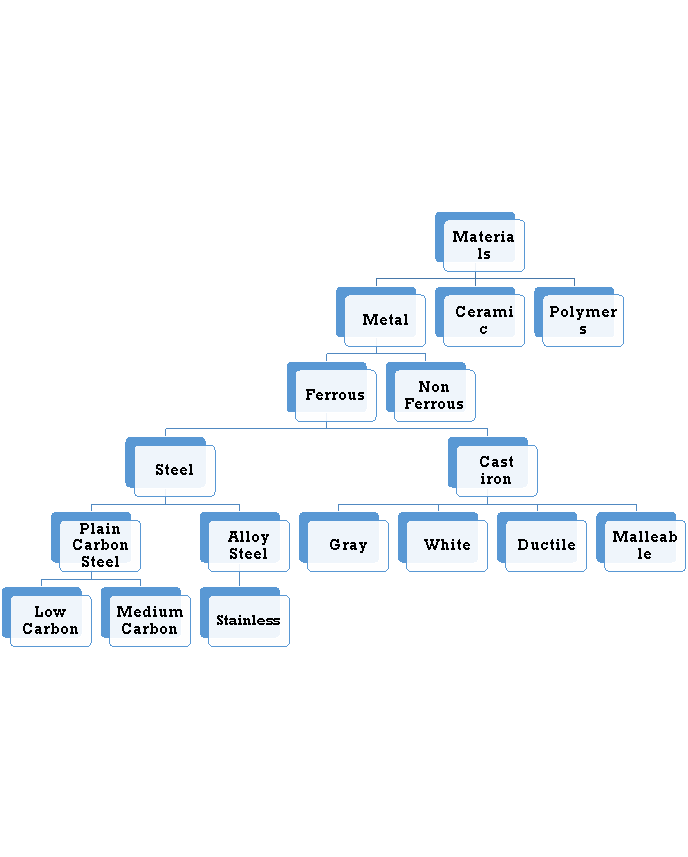
The combinations of metallic elements, such as iron, titanium, aluminum, and gold, which may also contain small amounts of non-metallic elements, such as carbon, nitrogen, and oxygen are metallic materials. Metals are mixed with other elements to form an alloy instead of using pure. This is usually necessary to obtain the required properties of the material. The factors that effect the choice of metals and alloys as biomaterials are:
physical and mechanical properties
degradation of the material
biocompatibility
6.1.2 Alloy Definition and Classification
It is the mixture of metallic solid solution that composed of two or more elements. E.g.: Brass, pewter, phosphor bronze, steel. Alloys typically refer to metals that are formed from the mixture of two or more elements. One of those elements must be a metallic element, but other constituents may not always be metallic. Alloys give single solid phase microstructure. The partial solutions give two or more than those phases that may or may not be the homogenous in distribution. The classification of alloys on the basis of their composition is:
Ferrous Alloys
Non Ferrous Alloys
Ferrous Alloys: Ferrous alloys are metals that consist mostly of iron that is Fe. Steel is an iron-based alloy containing typically less than 1% carbon, where iron frequently contains 2% or more carbon. They are produced in larger quantities than any other metallic material. Their mechanical properties can be improved by heat treating and, in the case of steels, by working. Stainless steels were developed to resist corrosion and generally contain 12% or more chromium, and may contain nickel in any amount up to or even exceeding the chromium content based upon the mechanical properties desired and application.
|
Non Ferrous Alloys: A metal is defined as non-ferrous it means that it does not have a significant amount of iron in its chemical composition. That means nearly all metal alloys have some trace, or non-significant, amount of iron in their composition. This does not make them ferrous alloys though. Non-ferrous alloys generally have iron compositions of less than one percent as measured by weight. If iron constitutes a large percentage of a metal, such as if it is the first or second most abundant element in the metal’s chemical composition, then the metal is considered ferrous.
6.1.3 Purposes of making alloys
Alloys enhance the metal hardness: The pure metal posses the property of softness. The hardness of a metal can be enhanced by alloying it with another metal or nonmetal.
Alloys help in decreasing the melting point: Pure metals have a high melting point. The melting point lowers when pure metals are alloyed with other metals or nonmetals. This makes the metals easily fusible. This property is utilized to make useful alloys called solders.
Alloy help in enhancing the tensile strength: Alloy formation increases the tensile strength of the parent metal.
Alloy help in enhancing corrosion resistance: They are more resistant to corrosion than pure metals. Metals in pure form are chemically reactive and can be easily corroded by the surrounding atmospheric gases and moisture. Alloying a metal increases the inertness of the metal, which, in turn, increases corrosion resistance.
Modify color: The color of pure metal can be modified by alloy with other metals or nonmetals containing suitable color pigments.
Provide better castability: One of the most essential requirements of getting good castings is the expansion of the metal on solidification. Pure molten metals undergo contraction on solidification.
6.1.4 Ferrous alloys
Ferrous alloys are metals that consist mostly of iron that is Fe. Steel is an iron-based alloy containing typically less than 1% carbon, where iron frequently contains 2% or more carbon. They are produced in larger quantities than any other metallic material. Their mechanical properties can be improved by heat treating and, in the case of steels, by working. Stainless steels were developed to resist corrosion and generally contain 12% or more chromium, and may contain nickel in any amount up to or even exceeding the chromium content based upon the mechanical properties desired and application.
|
6.1.5 Plain carbon steels (mild medium and high)
Plain carbon steels are iron-carbon alloys in which the properties are primarily derived from the presence of carbon. Some incidental elements like manganese, silicon, sulphur and phosphorus are present in small amounts due to the method of making steels and, not to modify the mechanical properties.
Alloy steels are those steels when, one, or more of the alloying elements are intentionally added to plain carbon steels to enhance, or induce some property, or properties. It is a bit difficult to make a clear cut distinction between plain carbon and alloy steel.
However, AISI (American Iron and Steel Institute) adopted the following definition. ‘Carbon steels are regarded as steels-containing not more than 1.65% manganese, 0.60% silicon and 0.60% copper, all other steels being regarded as alloy steels. Common alloying elements are nickel, chromium, vanadium, silicon, manganese, etc.
6.1.6 Stainless steels
Stainless steel is a family of alloy steels usually containing 10 to 30% of chromium. In conjunction with low carbon content, chromium imparts remarkable resistance to corrosion and heat. Other elements such as nickel, molybdenum, titanium, aluminium, niobium, copper, nitrogen, phosphorus or selenium, may be added to increase corrosion resistance to specific environments, enhance oxidation resistance, and impart special characteristics.
Properties of Stainless Steel:
Corrosion resistant.
High tensile strength.
Very durable.
Temperature resistant.
Easy formability and fabrication.
Low-maintenance
Attractive appearance.
Environmentally friendly
6.1.7 Nonferrous alloys
A metal is defined as non-ferrous it means that it does not have a significant amount of iron in its chemical composition. That means nearly all metal alloys have some trace, or non-significant, amount of iron in their composition. This does not make them ferrous alloys though. Non-ferrous alloys generally have iron compositions of less than one percent as measured by weight. If iron constitutes a large percentage of a metal, such as if it is the first or second most abundant element in the metal’s chemical composition, then the metal is considered ferrous.
6.1.8 Copper alloy
Copper alloys are those metal alloys that have copper as their principal component. They possess high resistivity against corrosion. The best known traditional types are bronze; where tin is a significant addition, and brass, using zinc instead. Both of these are imprecise terms, having both been commonly referred to as lattens in the past.
Applications:
Power transmission lines
Architectural applications
Cooking utensils
Spark plugs
Electrical wiring, cables and busbars
High conductivity wires
Electrodes
Heat exchangers and refrigeration tubing
Plumbing
Water-cooled copper crucibles.
Roofing
Cladding
Rainwater systems
Heating systems
Water pipes and fittings
Oil and gas lines
Electrical wiring.
6.1.9 Nickel alloy (Nichrome)
Nichrome is an alloy of nickel and chromium that consists of some amount of iron and other elements usually, 80% Ni and 20% Cr is present.
Nichrome is used in resistance wire. It is used in a very wide variety of devices where electric heating is required. It is used in some dental restorations (fillings). It is used in the explosives and fireworks industry as a bridge wire in electric ignition systems, such as electric matches and model rocket igniters.
Properties of Nichrome:
Nickel alloys resist high pressures and temperatures, making them well-suited for high-performance applications such as jet-engine blades. They also resist corrosion. That is why monel is used in deep-seal mining, where seawater poses a constant threat of corrosion.
Nickel and nickel alloys are non-ferrous metals with high strength and toughness, excellent corrosion resistance, and superior elevated temperature properties. Pure nickel is a bright silver-white metallic element of the iron group and is hard, malleable, and ductile. Pure nickel itself is tough and corrosion resistant and provides an excellent base for developing specialized alloys.
6.1.10 Aluminium alloy (Duralumin and Alnico)
Alnico:
Alnico is the name for an iron alloy that primarily consists of iron, aluminium, nickel & cobalt. Alnico alloys have ferromagnetic properties which makes it strong permanent magnets. These magnets also show excellent stability in a wide range temperature. There are effective in temperatures upto 1000∘F.
Composition of alnico is
1. Al (Aluminium)
2. Ni (Nickel)
3. Co (Cobalt)
sometimes it also includes titanium
Duralium:
Duralium is a metal consist of an alloy of aluminium, copper, magnesium and manganese. Duralium is a special kind of metal, it is hard made by subjecting it to heat treatment. It may be well spun, tempered, riveted, welded or machinated. The duralumin, which is effectively given heat treatment, can be effectively being resistant to corrosion. It can carry heavy loads, and is ductile. It is specially suited for aircraft construction.
Composition of duralumin
1. Cu (Copper)
2. Mg (Magnesium)
3. Mn (Manganese)
6.2.1 Definition
The term used for green chemistry is sustainable chemistry. The area of chemistry which deals with the design of products that reduces the generation of hazardous products. Green chemistry deals with improving the environment, it reduces the chemical action on the environment it includes the consumption of non-renewable resources. “Green Chemistry is the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products.”
6.2.2 Twelve principles of Green Chemistry
Principles of Green Chemistry:
There are following 12 major principles of green chemistry:
Preventing Waste
Maximize atom economy
Less hazardous chemical syntheses
Designing safer chemicals
Safer solvents and Auxiliaries
Increases Energy efficiency
Uses renewable feedstock
Reduce derivatives
Use catalysts, not stoichiometric reagent
Design chemicals and products to be degraded after use
Real time analysis to prevent pollution
Minimize the potential for accidents
UNIT 6
Metallic materials and Green Chemistry
6.1.1 Introduction
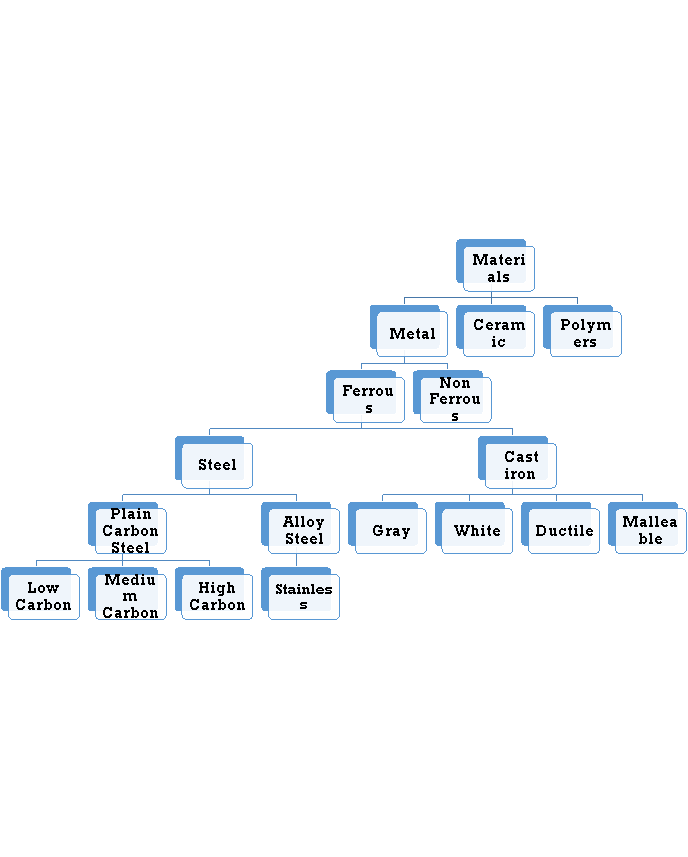
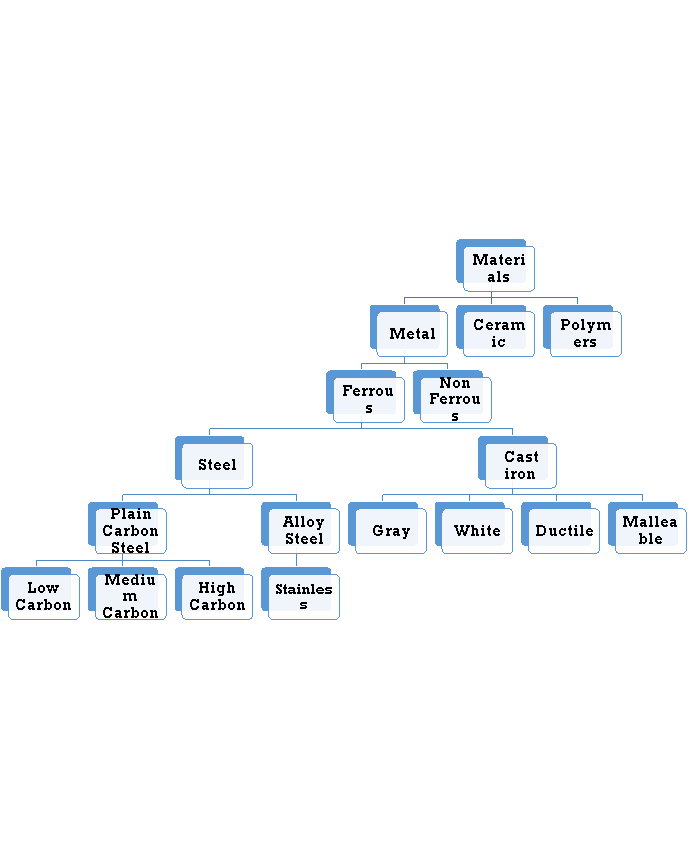
The combinations of metallic elements, such as iron, titanium, aluminum, and gold, which may also contain small amounts of non-metallic elements, such as carbon, nitrogen, and oxygen are metallic materials. Metals are mixed with other elements to form an alloy instead of using pure. This is usually necessary to obtain the required properties of the material. The factors that effect the choice of metals and alloys as biomaterials are:
physical and mechanical properties
degradation of the material
biocompatibility
6.1.2 Alloy Definition and Classification
It is the mixture of metallic solid solution that composed of two or more elements. E.g.: Brass, pewter, phosphor bronze, steel. Alloys typically refer to metals that are formed from the mixture of two or more elements. One of those elements must be a metallic element, but other constituents may not always be metallic. Alloys give single solid phase microstructure. The partial solutions give two or more than those phases that may or may not be the homogenous in distribution. The classification of alloys on the basis of their composition is:
Ferrous Alloys
Non Ferrous Alloys
Ferrous Alloys: Ferrous alloys are metals that consist mostly of iron that is Fe. Steel is an iron-based alloy containing typically less than 1% carbon, where iron frequently contains 2% or more carbon. They are produced in larger quantities than any other metallic material. Their mechanical properties can be improved by heat treating and, in the case of steels, by working. Stainless steels were developed to resist corrosion and generally contain 12% or more chromium, and may contain nickel in any amount up to or even exceeding the chromium content based upon the mechanical properties desired and application.
|
Non Ferrous Alloys: A metal is defined as non-ferrous it means that it does not have a significant amount of iron in its chemical composition. That means nearly all metal alloys have some trace, or non-significant, amount of iron in their composition. This does not make them ferrous alloys though. Non-ferrous alloys generally have iron compositions of less than one percent as measured by weight. If iron constitutes a large percentage of a metal, such as if it is the first or second most abundant element in the metal’s chemical composition, then the metal is considered ferrous.
6.1.3 Purposes of making alloys
Alloys enhance the metal hardness: The pure metal posses the property of softness. The hardness of a metal can be enhanced by alloying it with another metal or nonmetal.
Alloys help in decreasing the melting point: Pure metals have a high melting point. The melting point lowers when pure metals are alloyed with other metals or nonmetals. This makes the metals easily fusible. This property is utilized to make useful alloys called solders.
Alloy help in enhancing the tensile strength: Alloy formation increases the tensile strength of the parent metal.
Alloy help in enhancing corrosion resistance: They are more resistant to corrosion than pure metals. Metals in pure form are chemically reactive and can be easily corroded by the surrounding atmospheric gases and moisture. Alloying a metal increases the inertness of the metal, which, in turn, increases corrosion resistance.
Modify color: The color of pure metal can be modified by alloy with other metals or nonmetals containing suitable color pigments.
Provide better castability: One of the most essential requirements of getting good castings is the expansion of the metal on solidification. Pure molten metals undergo contraction on solidification.
6.1.4 Ferrous alloys
Ferrous alloys are metals that consist mostly of iron that is Fe. Steel is an iron-based alloy containing typically less than 1% carbon, where iron frequently contains 2% or more carbon. They are produced in larger quantities than any other metallic material. Their mechanical properties can be improved by heat treating and, in the case of steels, by working. Stainless steels were developed to resist corrosion and generally contain 12% or more chromium, and may contain nickel in any amount up to or even exceeding the chromium content based upon the mechanical properties desired and application.
|
6.1.5 Plain carbon steels (mild medium and high)
Plain carbon steels are iron-carbon alloys in which the properties are primarily derived from the presence of carbon. Some incidental elements like manganese, silicon, sulphur and phosphorus are present in small amounts due to the method of making steels and, not to modify the mechanical properties.
Alloy steels are those steels when, one, or more of the alloying elements are intentionally added to plain carbon steels to enhance, or induce some property, or properties. It is a bit difficult to make a clear cut distinction between plain carbon and alloy steel.
However, AISI (American Iron and Steel Institute) adopted the following definition. ‘Carbon steels are regarded as steels-containing not more than 1.65% manganese, 0.60% silicon and 0.60% copper, all other steels being regarded as alloy steels. Common alloying elements are nickel, chromium, vanadium, silicon, manganese, etc.
6.1.6 Stainless steels
Stainless steel is a family of alloy steels usually containing 10 to 30% of chromium. In conjunction with low carbon content, chromium imparts remarkable resistance to corrosion and heat. Other elements such as nickel, molybdenum, titanium, aluminium, niobium, copper, nitrogen, phosphorus or selenium, may be added to increase corrosion resistance to specific environments, enhance oxidation resistance, and impart special characteristics.
Properties of Stainless Steel:
Corrosion resistant.
High tensile strength.
Very durable.
Temperature resistant.
Easy formability and fabrication.
Low-maintenance
Attractive appearance.
Environmentally friendly
6.1.7 Nonferrous alloys
A metal is defined as non-ferrous it means that it does not have a significant amount of iron in its chemical composition. That means nearly all metal alloys have some trace, or non-significant, amount of iron in their composition. This does not make them ferrous alloys though. Non-ferrous alloys generally have iron compositions of less than one percent as measured by weight. If iron constitutes a large percentage of a metal, such as if it is the first or second most abundant element in the metal’s chemical composition, then the metal is considered ferrous.
6.1.8 Copper alloy
Copper alloys are those metal alloys that have copper as their principal component. They possess high resistivity against corrosion. The best known traditional types are bronze; where tin is a significant addition, and brass, using zinc instead. Both of these are imprecise terms, having both been commonly referred to as lattens in the past.
Applications:
Power transmission lines
Architectural applications
Cooking utensils
Spark plugs
Electrical wiring, cables and busbars
High conductivity wires
Electrodes
Heat exchangers and refrigeration tubing
Plumbing
Water-cooled copper crucibles.
Roofing
Cladding
Rainwater systems
Heating systems
Water pipes and fittings
Oil and gas lines
Electrical wiring.
6.1.9 Nickel alloy (Nichrome)
Nichrome is an alloy of nickel and chromium that consists of some amount of iron and other elements usually, 80% Ni and 20% Cr is present.
Nichrome is used in resistance wire. It is used in a very wide variety of devices where electric heating is required. It is used in some dental restorations (fillings). It is used in the explosives and fireworks industry as a bridge wire in electric ignition systems, such as electric matches and model rocket igniters.
Properties of Nichrome:
Nickel alloys resist high pressures and temperatures, making them well-suited for high-performance applications such as jet-engine blades. They also resist corrosion. That is why monel is used in deep-seal mining, where seawater poses a constant threat of corrosion.
Nickel and nickel alloys are non-ferrous metals with high strength and toughness, excellent corrosion resistance, and superior elevated temperature properties. Pure nickel is a bright silver-white metallic element of the iron group and is hard, malleable, and ductile. Pure nickel itself is tough and corrosion resistant and provides an excellent base for developing specialized alloys.
6.1.10 Aluminium alloy (Duralumin and Alnico)
Alnico:
Alnico is the name for an iron alloy that primarily consists of iron, aluminium, nickel & cobalt. Alnico alloys have ferromagnetic properties which makes it strong permanent magnets. These magnets also show excellent stability in a wide range temperature. There are effective in temperatures upto 1000∘F.
Composition of alnico is
1. Al (Aluminium)
2. Ni (Nickel)
3. Co (Cobalt)
sometimes it also includes titanium
Duralium:
Duralium is a metal consist of an alloy of aluminium, copper, magnesium and manganese. Duralium is a special kind of metal, it is hard made by subjecting it to heat treatment. It may be well spun, tempered, riveted, welded or machinated. The duralumin, which is effectively given heat treatment, can be effectively being resistant to corrosion. It can carry heavy loads, and is ductile. It is specially suited for aircraft construction.
Composition of duralumin
1. Cu (Copper)
2. Mg (Magnesium)
3. Mn (Manganese)
6.2.1 Definition
The term used for green chemistry is sustainable chemistry. The area of chemistry which deals with the design of products that reduces the generation of hazardous products. Green chemistry deals with improving the environment, it reduces the chemical action on the environment it includes the consumption of non-renewable resources. “Green Chemistry is the utilization of a set of principles that reduces or eliminates the use or generation of hazardous substances in the design, manufacture and application of chemical products.”
6.2.2 Twelve principles of Green Chemistry
Principles of Green Chemistry:
There are following 12 major principles of green chemistry:
Preventing Waste
Maximize atom economy
Less hazardous chemical syntheses
Designing safer chemicals
Safer solvents and Auxiliaries
Increases Energy efficiency
Uses renewable feedstock
Reduce derivatives
Use catalysts, not stoichiometric reagent
Design chemicals and products to be degraded after use
Real time analysis to prevent pollution
Minimize the potential for accidents