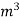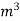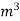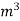UNIT – IV
FUELS
Definition: - A fuel is a substance that contains carbon and hydrogen undergoes combustion in presence of oxygen to gives large amount of energy.
 Fuel + O2 CO2 + H2O + Energy
Fuel + O2 CO2 + H2O + Energy
Fuels:-
A fuel is the substance which on combustion produces a large amount of heart.
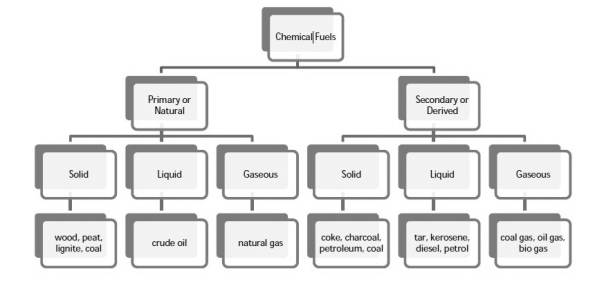
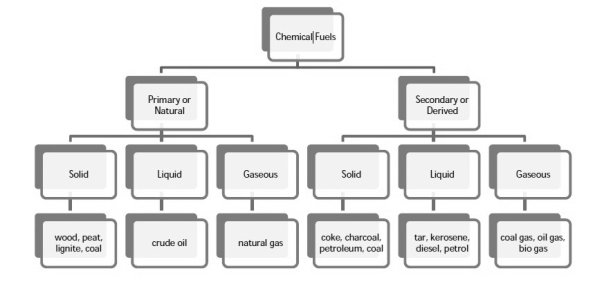
Chemical fuels are classified on the basis of occurrence into :-
- Primary ( natural )
- Secondary (derived ) types / manmade .
The secondary fuels are obtained from primary fuel by processing or they are manmade. e.g.:- charcoal is obtained from wood by partial combustion of wood , ethyl alcohol is obtained by fermentation of carbohydrates .
Both the primary and secondary fuels are further classified on the basis of physical state into solid liquid and gaseous fuels.
|
Extra definitions:-
1] Fuels:-
Fuels are defined as materials that create usable energy through chemical nuclear or electrochemical reaction.
2] Chemical fuel:-
The fossil fuels, wood vegetal oils etc. . which produce heat on burning are known as chemical fuels.
Comparison between solid, liquid, and gaseous fuels.
SOLID FUELS | LIQUID FUELS | GASEOUS FUELS |
|
|
|
|
|
|
Calorific value : Efficiency of combustible fuels can be measured in terms of their calorific value . It is the heat evolved on combustion of a unit quantity of fuel.
4.4. Definition
- It is defined as the amount of heat obtained on complete combustion of unit mass of a solid or liquid fuel or unit volume of a gaseous fuel at STP.
System | Solid or liquid fuel | Gaseous fuel |
CGS | Cal / gm | Cal / liter |
MKS | Kcal / kg | Kcal / |
S.I | Joules / kg | Joules / |
Biodiesel can be used as a good fuel for diesel engine but generally it is used as its 20 percent mixture with diesel.
- Biodiesel is cheaper
2. It has high c.v of about 40 kg / gm
3. It is regenerative and environmentally friendly .
4. It does not give out particulate and CO pollutants .
5. It has certain extent of lubricity
6. It uses provided good market to vegetable oils and reduces our dependence on biodiesel on foreign countries , saving currency.
7. It is clean to use biodiesel in diesel engine .
SOLID FUELS | LIQUID FUELS | GASEOUS FUELS |
|
|
|
|
|
|
Higher Calorific Value
- Gross calorific value of a fuel can be defined as the total amount of heat obtained on complete combustion of unit mass of a solid or liquid fuel or unit volume of a gaseous fuel ( S T P ) and on cooling the products of combustion to 15 degree c. The gross calorific value is also called as higher calorific value.
- The G.V.C is of only theoretical importance because in actual practice we do not have any provision of cooling the products of combustion during combustion of fuel in an engine, furnace or any other fuel burning device.
Lower Calorific Value:
- Net calorific value is defined as the amount of heat obtained practically on complete combustion of unit mass of solid or liquid fuel or unit mass of solid or liquid fuel or unit volume of a gaseous fuel at step and the products of combustion are allowed to escape with some heat N.C.V is also called as lower calorific value.
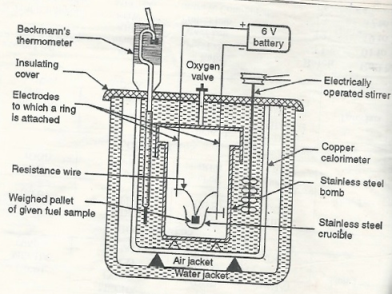
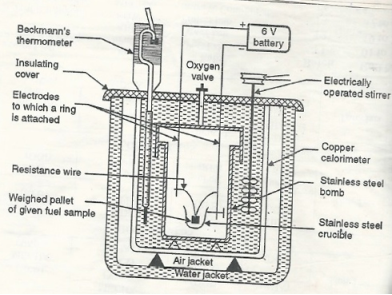
Bomb Calorimeter
The gross calorific value of solid fuels and liquid fuels can be determined by bomb calorimeter. (If the liquid is volatile, then it is filled in a polythene capsule of negligible mass then used in experiment.
Construction: A bomb colorimeter consists of
- Bomb pot
- Calorie meter
- Water and air jackets
- Accessories
- Pellet press
- Oxygen cylinder
Bomb pot :-
It is a cylindrical strong stainless-steel pot having a lid . the lid can be fitted air. Tight to bomb pot by screwing.
- There are two type of electrodes fitted through lid and there is an oxygen inlet valve as its center.
- One of these electrodes is provided with a ring to hold the crucible containing fuel .there is thin resistance wire tied to the electrodes in loop form and the loop touches the fuel.
- The weighed fuel is burnt in the bomb pot in the presence of high-pressure oxygen.
Calorimeter :-
- There is a stainless steel or copper calorimeter in which the bomb pot is kept .it contains a known volume of water and the water is kept circulating around the bomb pot with the help of a stirrer.
- A Beckman thermometer or digital thermometer is kept in the water of calorimeter , which can record the rise in temperature of welter due to absorb in a heat generated .
- There are insulator stands between calorimeter and water jacket.
Accessories :-
- There is a pellet press to convert the powder of solid fuel to pellet form .for a liquid fuel a capsule of negligible weight can be used.
- There is an oxygen cylinder with pressure gauge to fill oxygen in the bomb pot at the pressure of nearly 25 kg / cm².
- There is also a D.C battery of a about 6 volts to start combustion of fuel.
|
Fig: Bomb Caloriemeter
Working :-
- Weigh the pellet of solid fuel or liquid capsule and keep it in the crucible .keep the crucible in the ring of the electrode .keep the resistance wire touching to the fuel.
- Add about 10 ml of distilled water at the bottom of bomb pot and fix the lid tightly to bomb by screwing.
- Fill the bomb with oxygen at the pressure about 25 kg / cm².
- Place the bomb in calorimeter add known volume of water in the calorimeter so that the bomb gets immersed in the water.
- Place the calorimeter in the water jacket over the plastic studs .keep the thermometer and stirrer in the water of calorimeter.
- Put the plastic cover on the and make electrical connections from battery to electrodes.
- Operate the stirrer for s minutes and note the initial temperature of water ( t1° c ).
- Pass the current for about 5 – 10 seconds to heat the wire so that the fuel catches fire. If the fuel contains S and N elements, they get converted to SO3 and N2O5 .these gases get dissolved in the distilled water in bomb to form H2SO4 and HNO3 ( along with liberation little heat ).
- Note the maximum temperature reached .after that note the rate of fall of temperature per minute and the time taken for reaching to initial temp. are noted.
- Open the bomb pot and wash the contents at its bottom into a beaker to find out the amount of H2SO4 and HNO3 formed.
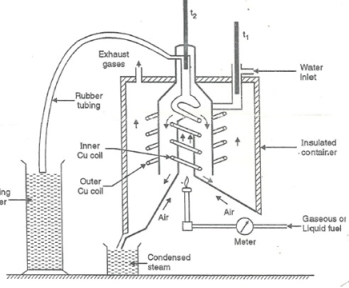
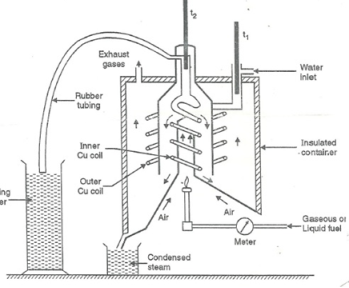
Boy’s Calorimeter:
Principle :-
The gaseous fuel is burnt at a known constant rate in the calorimeter under such conditions that entire amount of heat produced is absorbed by water.
This calorimeter is used to measure calorific value of gaseous fuels and highly volatile fuels.
- Gas burner
- Combustion chamber ( chimney )
- Thermometers
- Insulating cover
- Gas burner :-
- There is a gas burner in which a known volume of gas is burnt at a known pressure .the gas is burnt at the rate of 3 – 4 lit. per minute.
3. Combustion chamber ( chimney ) :-
Around the burner there Is a combustion chamber which has a copper tubing coiled inside as well as outside of it water enters from top of the outer coils , moves to bottom of chimney and then goes up through the inner coil to the exit at top.
4. Thermometer :-
There are two thermometer to measure temperatures of inlet water and outlet water.
5. Insulating cover :-
The assembly is covered with by an insulator to detach combustion chamber from atmosphere .there are three holes for exhaust gas, water inlet and condensed steam.
Working :-
- Start burning the gas at suitable pressure and adjust the rate of water flow such that the temperature of outgoing water remains constant.
- Burn the gas for 5 – 10 minutes to have the steady temperatures in and around the combustion chamber.
- After the steady ( temperature in and around the combustion chamber ) conditions of outgoing.
A) Volume of gas burnt at given temperature and pressure in certain time period.
B) Quantity of water passed through coil during this period.
C) Mass of water condensed from product gas during the period.
D) The steady rise in temperature of water ( t2 – t1 )
|
Fig: Boy’s gas calorimeter
Calculations :-
- First convert the volume of gas burnt to volume of gas at STP .let this STP volume be V
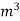 .
. - Let W = mass of cooling water used in the period of observation in Kg
- Let m= mass of water condensate in kg
- L= G.C.V OF THE FUEL
Heat produced by combustion of fuel = heat absorbed by cooling water
( assuming no heat loss in the steady state conditions )
Vl = w ( t2 – t1 )
L = 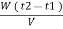 Kcal /
Kcal / 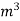
The mass of condensate water per m of gas will be m/v kg /m .
If this water had left as steam in product gases it would have taken away heat.
= 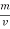 *587 kcal /
*587 kcal / 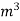
Therefore ,
NCV = GCV - 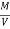 *587
*587
NCV = 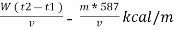
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A 1.000 g sample of octane (C8H18) is burned in a bomb calorimeter containing 1200 grams of water at an initial temperature of 25.00ºC. After the reaction, the final temperature of the water is 33.20ºC. The heat capacity of the calorimeter (also known as the “calorimeter constant”) is 837 J/ºC. The specific heat of water is 4.184 J/g ºC. Calculate the heat of combustion of octane in kJ/mol.
Since this is a combustion reaction, heat flows from the system to the surroundings- thus, it is exothermic. The heat released by the reaction will be absorbed by two things: (a) the water in the calorimeter and (b) the calorimeter itself.
- Calculate the heat absorbed by the water (qwater)
m = 1200 grams
cwater = 4.184 J/gºC
∆T = 33.20 – 25.00 = 8.20ºC
qwater = (m)(c)(∆T), so
qwater = (1200 g)( .4 184)( .8 20 C)
=41170.56 J
= 41.2 kJ ⋅
b. Calculate the heat absorbed by the calorimeter (qcal)
The temperature change of the calorimeter is the same as the temperature change for water. In this step, however, we must use the heat capacity of the calorimeter, which is already known. When using heat capacity, the mass of the calorimeter is not required for the calculation. (It’s already incorporated into the heat capacity).
Ccal = 837 J/ºC
∆T = 33.20 – 25.00 = 8.20ºC
qcal = (Ccal)(∆T),
so,
qcal = (837 )( .8 20 C) = 6863 J 4. = 6.86 kJ
UNIT – IV
FUELS
Definition: - A fuel is a substance that contains carbon and hydrogen undergoes combustion in presence of oxygen to gives large amount of energy.
 Fuel + O2 CO2 + H2O + Energy
Fuel + O2 CO2 + H2O + Energy
Fuels:-
A fuel is the substance which on combustion produces a large amount of heart.
Chemical fuels are classified on the basis of occurrence into :-
- Primary ( natural )
- Secondary (derived ) types / manmade .
The secondary fuels are obtained from primary fuel by processing or they are manmade. e.g.:- charcoal is obtained from wood by partial combustion of wood , ethyl alcohol is obtained by fermentation of carbohydrates .
Both the primary and secondary fuels are further classified on the basis of physical state into solid liquid and gaseous fuels.
|
Extra definitions:-
1] Fuels:-
Fuels are defined as materials that create usable energy through chemical nuclear or electrochemical reaction.
2] Chemical fuel:-
The fossil fuels, wood vegetal oils etc. . which produce heat on burning are known as chemical fuels.
Comparison between solid, liquid, and gaseous fuels.
SOLID FUELS | LIQUID FUELS | GASEOUS FUELS |
|
|
|
|
|
|
Calorific value : Efficiency of combustible fuels can be measured in terms of their calorific value . It is the heat evolved on combustion of a unit quantity of fuel.
4.4. Definition
- It is defined as the amount of heat obtained on complete combustion of unit mass of a solid or liquid fuel or unit volume of a gaseous fuel at STP.
System | Solid or liquid fuel | Gaseous fuel |
CGS | Cal / gm | Cal / liter |
MKS | Kcal / kg | Kcal / |
S.I | Joules / kg | Joules / |
Biodiesel can be used as a good fuel for diesel engine but generally it is used as its 20 percent mixture with diesel.
- Biodiesel is cheaper
2. It has high c.v of about 40 kg / gm
3. It is regenerative and environmentally friendly .
4. It does not give out particulate and CO pollutants .
5. It has certain extent of lubricity
6. It uses provided good market to vegetable oils and reduces our dependence on biodiesel on foreign countries , saving currency.
7. It is clean to use biodiesel in diesel engine .
SOLID FUELS | LIQUID FUELS | GASEOUS FUELS |
|
|
|
|
|
|
Higher Calorific Value
- Gross calorific value of a fuel can be defined as the total amount of heat obtained on complete combustion of unit mass of a solid or liquid fuel or unit volume of a gaseous fuel ( S T P ) and on cooling the products of combustion to 15 degree c. The gross calorific value is also called as higher calorific value.
- The G.V.C is of only theoretical importance because in actual practice we do not have any provision of cooling the products of combustion during combustion of fuel in an engine, furnace or any other fuel burning device.
Lower Calorific Value:
- Net calorific value is defined as the amount of heat obtained practically on complete combustion of unit mass of solid or liquid fuel or unit mass of solid or liquid fuel or unit volume of a gaseous fuel at step and the products of combustion are allowed to escape with some heat N.C.V is also called as lower calorific value.
Bomb Calorimeter
The gross calorific value of solid fuels and liquid fuels can be determined by bomb calorimeter. (If the liquid is volatile, then it is filled in a polythene capsule of negligible mass then used in experiment.
Construction: A bomb colorimeter consists of
- Bomb pot
- Calorie meter
- Water and air jackets
- Accessories
- Pellet press
- Oxygen cylinder
Bomb pot :-
It is a cylindrical strong stainless-steel pot having a lid . the lid can be fitted air. Tight to bomb pot by screwing.
- There are two type of electrodes fitted through lid and there is an oxygen inlet valve as its center.
- One of these electrodes is provided with a ring to hold the crucible containing fuel .there is thin resistance wire tied to the electrodes in loop form and the loop touches the fuel.
- The weighed fuel is burnt in the bomb pot in the presence of high-pressure oxygen.
Calorimeter :-
- There is a stainless steel or copper calorimeter in which the bomb pot is kept .it contains a known volume of water and the water is kept circulating around the bomb pot with the help of a stirrer.
- A Beckman thermometer or digital thermometer is kept in the water of calorimeter , which can record the rise in temperature of welter due to absorb in a heat generated .
- There are insulator stands between calorimeter and water jacket.
Accessories :-
- There is a pellet press to convert the powder of solid fuel to pellet form .for a liquid fuel a capsule of negligible weight can be used.
- There is an oxygen cylinder with pressure gauge to fill oxygen in the bomb pot at the pressure of nearly 25 kg / cm².
- There is also a D.C battery of a about 6 volts to start combustion of fuel.
|
Fig: Bomb Caloriemeter
Working :-
- Weigh the pellet of solid fuel or liquid capsule and keep it in the crucible .keep the crucible in the ring of the electrode .keep the resistance wire touching to the fuel.
- Add about 10 ml of distilled water at the bottom of bomb pot and fix the lid tightly to bomb by screwing.
- Fill the bomb with oxygen at the pressure about 25 kg / cm².
- Place the bomb in calorimeter add known volume of water in the calorimeter so that the bomb gets immersed in the water.
- Place the calorimeter in the water jacket over the plastic studs .keep the thermometer and stirrer in the water of calorimeter.
- Put the plastic cover on the and make electrical connections from battery to electrodes.
- Operate the stirrer for s minutes and note the initial temperature of water ( t1° c ).
- Pass the current for about 5 – 10 seconds to heat the wire so that the fuel catches fire. If the fuel contains S and N elements, they get converted to SO3 and N2O5 .these gases get dissolved in the distilled water in bomb to form H2SO4 and HNO3 ( along with liberation little heat ).
- Note the maximum temperature reached .after that note the rate of fall of temperature per minute and the time taken for reaching to initial temp. are noted.
- Open the bomb pot and wash the contents at its bottom into a beaker to find out the amount of H2SO4 and HNO3 formed.
Boy’s Calorimeter:
Principle :-
The gaseous fuel is burnt at a known constant rate in the calorimeter under such conditions that entire amount of heat produced is absorbed by water.
This calorimeter is used to measure calorific value of gaseous fuels and highly volatile fuels.
- Gas burner
- Combustion chamber ( chimney )
- Thermometers
- Insulating cover
- Gas burner :-
- There is a gas burner in which a known volume of gas is burnt at a known pressure .the gas is burnt at the rate of 3 – 4 lit. per minute.
3. Combustion chamber ( chimney ) :-
Around the burner there Is a combustion chamber which has a copper tubing coiled inside as well as outside of it water enters from top of the outer coils , moves to bottom of chimney and then goes up through the inner coil to the exit at top.
4. Thermometer :-
There are two thermometer to measure temperatures of inlet water and outlet water.
5. Insulating cover :-
The assembly is covered with by an insulator to detach combustion chamber from atmosphere .there are three holes for exhaust gas, water inlet and condensed steam.
Working :-
- Start burning the gas at suitable pressure and adjust the rate of water flow such that the temperature of outgoing water remains constant.
- Burn the gas for 5 – 10 minutes to have the steady temperatures in and around the combustion chamber.
- After the steady ( temperature in and around the combustion chamber ) conditions of outgoing.
A) Volume of gas burnt at given temperature and pressure in certain time period.
B) Quantity of water passed through coil during this period.
C) Mass of water condensed from product gas during the period.
D) The steady rise in temperature of water ( t2 – t1 )
|
Fig: Boy’s gas calorimeter
Calculations :-
- First convert the volume of gas burnt to volume of gas at STP .let this STP volume be V
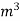 .
. - Let W = mass of cooling water used in the period of observation in Kg
- Let m= mass of water condensate in kg
- L= G.C.V OF THE FUEL
Heat produced by combustion of fuel = heat absorbed by cooling water
( assuming no heat loss in the steady state conditions )
Vl = w ( t2 – t1 )
L = 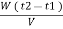 Kcal /
Kcal / 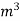
The mass of condensate water per m of gas will be m/v kg /m .
If this water had left as steam in product gases it would have taken away heat.
= 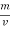 *587 kcal /
*587 kcal / 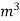
Therefore ,
NCV = GCV - 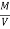 *587
*587
NCV = 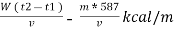
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
A 1.000 g sample of octane (C8H18) is burned in a bomb calorimeter containing 1200 grams of water at an initial temperature of 25.00ºC. After the reaction, the final temperature of the water is 33.20ºC. The heat capacity of the calorimeter (also known as the “calorimeter constant”) is 837 J/ºC. The specific heat of water is 4.184 J/g ºC. Calculate the heat of combustion of octane in kJ/mol.
Since this is a combustion reaction, heat flows from the system to the surroundings- thus, it is exothermic. The heat released by the reaction will be absorbed by two things: (a) the water in the calorimeter and (b) the calorimeter itself.
- Calculate the heat absorbed by the water (qwater)
m = 1200 grams
cwater = 4.184 J/gºC
∆T = 33.20 – 25.00 = 8.20ºC
qwater = (m)(c)(∆T), so
qwater = (1200 g)( .4 184)( .8 20 C)
=41170.56 J
= 41.2 kJ ⋅
b. Calculate the heat absorbed by the calorimeter (qcal)
The temperature change of the calorimeter is the same as the temperature change for water. In this step, however, we must use the heat capacity of the calorimeter, which is already known. When using heat capacity, the mass of the calorimeter is not required for the calculation. (It’s already incorporated into the heat capacity).
Ccal = 837 J/ºC
∆T = 33.20 – 25.00 = 8.20ºC
qcal = (Ccal)(∆T),
so,
qcal = (837 )( .8 20 C) = 6863 J 4. = 6.86 kJ