Unit 01
Water Treatment
1.1 Introduction:
Hardness can be defined as a soap consuming capacity of water sample. Soaps are sodium salts of fatty acids like oleic acid, palmetic acid and stearic acid. They dissolve readily .in water to form lather due to which it has cleansing property.


- But compounds of fatty acids with other metals done dissolve in water.
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd.
 2
2
(calciumstearate)
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd
- These other metal ions are responsible for the hardness of water most important metal of ions which cause hardness to water are calcium and magnesium ions.
- The hardness of water along can be calculated from the amount of calcium and magnesium ions present in water along with bicarbonates, sulphates chlorides and nitrates.
TYPES OF WATER | HARDNESS |
Soft | 0 – 75 |
Moderately hard | 75 – 150 |
Hard | 150 – 300 |
Very hard | Above 300 |
1.2 Effect of water on rocks and minerals:
The process by which rocks are broken down on the earth’s surface into such things as sediments, clays, soils and substances that are dissolved in water are called as the weathering. The weathering occurs when the earth’s crust is uplifted by tectonic forces. After the physical breakup and chemical decay of exposed rocks by weathering, the loosened rock fragments and alterations products are carried away through the process of erosion. Erosion relies on transporting agents such as wind, rivers, ice, snow and downward movement of materials to carry weathered products away from the source area. As weathered products are carried away, fresh rocks are exposed to further weathering. Over time, that mountain or hill is gradually worn down.
There are two different types of weathering:
(a) Chemical Weathering: Chemical weathering occurs by the chemical between minerals of rocks and external agents like air or water. Oxygen oxidizes minerals to alteration products whereas water can convert minerals to clays or dissolve minerals completely.
(b) Physical Weathering: Physical weathering occurs when rocks are broken apart by mechanical processes such as rock fracturing, freezing and thawing, or breakage during transport by rivers or glaciers.
Factors Which Control the Rates of Weathering
1. The mineralogy and structure of a rock affects weathering.
2. Different minerals weather at different rates.
3. A rock’s structure also affects its susceptibility to weathering.
Climate
1. Rainfall and temperature can affect the rate in which rocks weather. High temperatures and greater rainfall increase the rate of chemical weathering.
2. Rocks in tropical regions exposed to abundant rainfall and hot temperatures weather much faster than similar rocks residing in cold, dry regions.
Soil
1. Soils affect the rate in which a rock weathers. Soils retain rainwater so that rocks covered by soil are subjected to chemical reactions with water much longer than rocks not covered by soil.
2. Minerals in a rock buried in soil will therefore break down more rapidly than minerals in a rock that is exposed to air.
Chemical Weathering
Chemical weathering is a process where minerals in a rock may be converted into clays, oxidized or simply dissolved. E.g.:
(a) Silicate into clay conversion
(b) Mineral dissolving
(c) Oxidation
1.3 Hardness of water:
Hard water: is water that contains an required quantity of dissolved minerals (like calcium and magnesium) As rainwater falls, it is naturally soft. However, as water flows through the ground and into waterways, it picks up minerals like chalk, lime and mostly calcium and magnesium and becomes hard water. Since hard water contains essential minerals, it is essentially used as drinking water. Not only because of the health benefits, but also the flavour. Water that does not produce lather with soap readily is called hard water. Water hardness is usually measured as calcium hardness in milligrams per litre (mg/l) OR parts per million (ppm) OR in grains per gallon (GPG).
For eg: sea water, river water, spring water, lake water and well water.
1.4 Disadvantages of hard water:
Disadvantages of using hard water are-
1. Hard water is unfit for washing as it is difficult to form lather with soap.
2. Scum may form in a reaction with soap, wasting the soap.
3. Furring of tea kettles will take place due to the formation of carbonates of calcium and magnesium.
4. Hard blocks hot water pipes. This is due to the formation of layers of carbonates of calcium and magnesium.
1.5 Boiler feed water – scale and sludge formation in boilers – caustic embrittlement – boiler corrosion – priming and foaming:
The water that is fed into the boiler for the production of steam is called boiler feed water. The fed water into the boiler must be free from turbidity, free from oil, alkalis and most important things the hardness causing substances. The used water are contaminated in maximum time. If the hard water is used then it leads to 4 major problems that are-
- Scale & Sludge Formation
- Primming and Foaming
- Caustic Embrittlement
- Boiler Corrosion
Scale & Sludge Formation: The water evaporates continuously and the dissolved salts concentration increases progressively. When their concentrations reach at saturation point, they are thrown out of water in precipitate form which get stick in inner walls of boiler. If the precipitation takes place in the form of loose or slimy precipitate it is called sludge. While if the precipitated matter forms a hard adhering coating on inner walls of boiler, then it is called as scale. Eg- MgCO3, MgCl2, MgSO4 etc.
Priming & Foaming: The duration at which boiler is producing steam rapidly, some particles of the condensed liquid are carried along with the steam. The process of wet steam formation is called priming. Priming is mainly caused by the presence of large amounts of dissolved solids, high steam velocities, sudden boiling etc. Whereas the continuous production of foam or bubbles in boilers which do not break easily are called as the foaming. This is caused due to the presence of substance like oils in water that reduce the surface tension in water.
Caustic Embrittelment: The use of high alkaline water in the boiler cause rust in the boiler which is called as Caustic Embrittelment. The presence of sodium carbonate plays a major role during the softening process.
Na2CO3 + H2O → NaOH + CO2
the caustic embrittelment is caused by using sodium phosphate as a softening agent instead of sodium carbonate.
Boiler Corrosion: The decay of the boiler material by attack of chemicals or electro-chemicals at its environment is called as boiler corrosion.
Reasons of boiler corrosion:
(i) Dissolved Oxygen
(ii) Dissolved Carbon di oxide
(iii) Acid from dissolved salts
Sludge, scale, priming and foaming, caustic embrittlement, boiler corrosion are collectively known as boiler troubles.
1.6 Softening methods-lime soda, zeolite and ion exchange process:
Lime Soda:
Surface water hardly exceed hardness level above 200 mg/1 and softening is not at all required in most of the cases, unless the water is being polluted by some effluent sources. In case of groundwater, hardness level of more than 1000 mg/1 are quite common. Since, soft water is corrosive, therefore public water supply are usually not softened below 30 to 50 mg/1. The most accepted and commonly used water softening methods are cat ion exchange and precipitation method. In order to obtain maximum profit, the factors to be considered are a good choice of a softening process, quality of the raw water, the cost of softening chemicals and the cost of disposing of waste streams.
Precipitation methods
The principle that follows the precipitation method is to bind calcium cations Ca and magnesium cations Mg , with ions of CO3 and OH . The precipitate CaC03 and Mg (OH)2 formed are removed from the water. Slake lime Ca(OH)2, Quick lime CaO , soda ash NaC03 and sodium hydroxide (caustic soda) NaOH, are reagents that are commonly used in water softening. Depending upon the quality of initial water, the following main precipitation methods are determined. a) Lime softening b) Lime - Soda softening c) Sodium Hydroxide softening Lime affects the carbonate hardness (alkalinity) and therefore can be used in order to decrease the carbonate hardness present in the initial water. This method however does not result in deep softening. Magnesium is removed from water if there is excess of OH” present. Water dissolved carbon dioxide is removed, total solids in the treated water diminishes and the total hardness in the lime treated water also reduces. But the pH increases to 10 or beyond. When lime is added to the hard water following reactions occurs, In the above reactions,
Lime Addition:-
Hardness Lime Precipitate
CO2 + Ca(OH)2 -- > CaCO3 + H2O Ca(HCO3)2 + Ca(OH)2 -- > 2CaCO3 + 2H2O Mg(HCO3)2 + Ca(OH)2 -- > CaCO3 + MgCO3 + 2H2O MgCO3 + Ca(OH)2 -- > CaCO3 + Mg(OH)2 CO2 the insoluble products do not contribute to the hardness, but It reacts with the lime, and thereby uses up some lime before the lime can start removing the hardness in water. Lime - Soda softening method is commonly practiced in most of the Public water supply. (Belan1984) The method is universal as water of almost any composition is treated with lime and soda. In this treatment, two reagents are used namely lime and soda ash. Lime as earlier discussed , decreases the carbonate hardness, (Mg2+) and removes C02 from the water.
Soda therefore reduces the non - carbonate hardness, mainly due to Ca2+, that shows after reaction with lime and the reaction occurs after the addition of soda ash is as follows.
Lime and Soda ash Addition:- Lime Precipitate MgSO4 + Ca(OH)2 -- > Mg(OH)2 + CaSO4 Soda ash Precipitate CaSO4 + Na2CO3 -- > CaCO3 + Na2SO4.
Zeolite Process:
Zeolite process is a process of softening hard water through ion exchange technique using a chemical compound zeolite. It possesses a chemical compound that has hydrated sodium aluminosilicate. Thus, the name of the process is called as zeolite process. Zeolite compound can exchange its sodium cations reversibly with calcium and magnesium ions in the water softening process.
There are two types of zeolite used in this process they include natural and synthetic zeolite. The natural form is found to be porous and synthetic form is a non-porous zeolite. However synthetic form possesses a high exchange capacity per unit weight than the natural form.
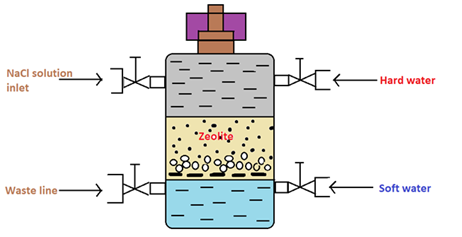
Figure 01: Cylinder Containing the Zeolite Bed
Process
In the water softening process, hard water is passed through a bed of zeolite (inside a cylinder) at a specified rate. Then the cations that cause the water-hardening will remain on the zeolite bed because these cations exchange with the sodium cations of zeolite. Therefore, the water coming out of this cylinder contains sodium cations rather than calcium and magnesium cations.
After some time, the zeolite bed gets exhausted, the water flow is stopped and treat the bed is treated with concentrated brine solution (10%) in order to regenerate the zeolite. The bed is treated with a brine solution, as it washes away all the calcium and magnesium ions, by exchanging them with sodium ions in a brine solution. Hence, this treatment regenerates the zeolite.
Ion Exchange Process:
Ion exchange or de ionization or de mineralization ion exchange resins are insoluble cross linked long chain organic polymers with a microporous structure and the functional group attached to the chains are responsible for the ion exchange properties resins contaning acidic function groups are capable of exchaning their anions with other anions which comes in their contact the ion exchange resins may be classified as :-
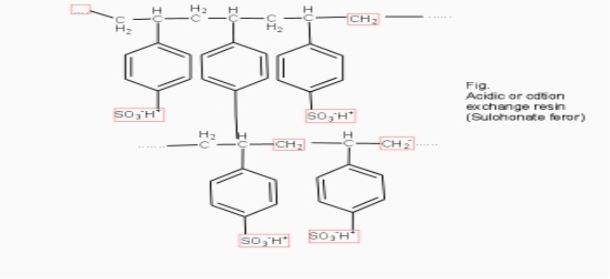
Cation exchange resins ( RH+) :-
They are mainly styrene divinly benzene co-polymers which on sulphonation or carbonoxylation become capable to exchange their hydrogen ions with the cations in the water.
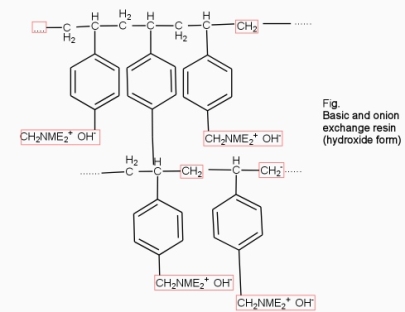
- Anion exchange resins :-
They are styrene – divinly benzene or amine fermaldehyde which contain amino or quaternary ammonium or4 quaternary phosphonium or tertiary sulphonium group as an integral part of the resin matrix.these after treatment with dilute NaOH solution become capable to exchange their OH- anions with anions in water .
Processes :-
The hard water is passed first through cation exchange column which removes all the cations like Ca²+ , Mg² + e.t.c . From it and equivalent amount of H+ ions are released from this column Water
2RH+ + Ca².+ ---------------------- R2Ca2+ + 2H
2RH+ Mg2+ --------------------------- R2Mg2+ 2H+
After cation exchange column the hard water passed through anion like cl- . Present in the water and equivalent amount of OH- ions are released from this column to water,
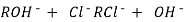
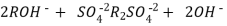
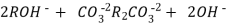
H+ And OH – ions ( released from cation exchange and anion exchange columns respectively ) get combined to produce water molecule.
H+ + OH -  H2O
H2O
Thus,
The water coming out form the exchanger is free from cations as well as an ions . Ion free water is known as de mineralizes water.
Regeneration :-
When capacities of cation and anion exchangers to exchange H+ and OH- ions respectively . Are lost they are then said to be exhausted.
The exhausted cation exchange column is regenerated by passing a solution of dil.HclH2SO4. The regeneration can be represented as
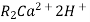

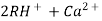 ( washing )
( washing )
The column is washed with deionized water and washing ( which contains Ca2+ and Cl2- ions ) is passed to sink or drain.
The exhausted anion exchange column is regenerated by passing a solution of dil. NaOH.
The column is washed with de-ionized water and washing (which contains NA+ and 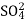 ions ) is pass to sink or drain.
ions ) is pass to sink or drain.
The regenerated ion exchange ion exchange resins are then used again.
Advantages :-
- The processes can be used to soften highly acidic or alkaline waters.
- It produces water of very low hardness ( say 2 ppm ) so it is very good for treating water for use in high pressure boilers.
Disadvantages :-
- The equipment is costly and more expensive chemicals are needed.
If water contains turbidity, then the out- put of the processes is reduced .the turbidity must be below 10 ppm if it is more it has to be removed first by filtration.
1.7 Specification of potable water and purification of Drinking water:
“Potable water” simply means water that is safe to drink, and it is becoming scarcer in the world. Increasing use is stressing freshwater resources worldwide, and a seemingly endless list of contaminants can turn once potable water into a health hazard or simply make it unacceptable aesthetically.
Of the more than 2 billion people who lack potable water at home, 844 million don’t have even basic drinking water service, including 263 million who must travel 30 minutes per trip to collect water. Unsafe drinking water is a major cause of diarrheal disease, which kills about 800,000 children under the age of 5 a year, usually in developing countries, but 90 countries are expected to fail to reach the goal of universal coverage by 2030. Physical requirement of potable water are-
S.No. | Characterstics | Treated Potable Water | Natural Potable Water | Method of Test |
1 | Color | 15 | 50 | ISO7887 |
2 | Turbidity | 5 | 25 | ISO7027 |
3 | PH | 6.5-8.5 | 5.5-9.5 | ISO10523 |
4 | Taste | - | - | - |
5 | Odor | Odorless | Odorless | - |
6 | Conductivity | 1500 | 2500 | ISO7888 |
7 | Suspended Matter | Non-deductable | Non-deductable | ISO11923 |
1.8 Chemical analysis of water-Hardness, acidity, alkalinity, chloride and dissolved oxygen:
Hardness:
Hardness is basically caused by the soluble salts of calcium, magnesium, iron, manganese, sodium, sulphates, chlorides and nitrates. The amount and type of impurity present in water define the degree of impurity in water. Hardness also depends on the amount of carbon-di-oxide present in the solution. Carbon-di-oxide influences the solubility of the impurities that cause hardness.
The hardness caused by carbonates and bicarbonates is called carbonate hardness. The hardness caused by all others components like chlorides, sulphates, nitrates etc is called non-carbonated hardness.
Temporary Hardness: It is caused by the presence of dissolved bicarbonates of calcium, magnesium and other heavy metals and the carbonate of iron. Temporary hardness is mostly destroyed by mere boiling of water, when bicarbonates are decomposed, will produce insoluble carbonates or hydroxides, which are deposited as a crust at the bottom of vessel.
Permanent Hardness: It occurs due to the presence of chlorides and sulphates of calcium, magnesium, iron, and other heavy metals. Unlike temporary hardness, permanent hardness is not destroyed on boiling.
The degree of hardness of drinking water has been classified in terms of the equivalent CaCO3 concentration as follows:
Soft | 0-60mg/L |
Medium | 60-120mg/L |
Hard | 120-180mg/L |
Very Hard | >180mg/L |
In a hard water sample, the total hardness can be determined by titrating the Ca2+ and Mg2+ present in an aliquot of the sample with Na2 EDTA solution, using NH4Cl-NH4OH buffer solution of pH 10 and Eriochrome Black-T as the metal indicator.
Na2H2Y (Disodium EDTA solution) → 2Na+ + H2Y-
Mg2+ + HD2- (blue) → MgD (wine red) + H+
D (metal-indicator complex, wine red colour) + H2Y- →Y- (metal EDTA complex colourless) + HD- (blue colour) + H+
Ethylenediamine tetra-acetic acid (EDTA) and its sodium salts form a chelated soluble complex when added to a solution of certain metal cations. If a small amount of a dye such as Eriochrome black T is added to an aqueous solution containing calcium and magnesium ions at a pH of 10 ± 0.1, the solution will become wine red. If EDTA is then added as a titrant, the calcium and magnesium will be complexed. After sufficient EDTA has been added to complex all the magnesium and calcium, the solution will turn from wine red to blue. This is the end point of the titration.
Units of Hardness:
1. Parts per million (ppm): Is the parts of calcium carbonate equivalent hardness per 106 parts of water, i.e, 1 ppm = 1 part of CaCO3 eq hardness in 106 parts of water.
2. Milligram per litre (mg/L): Is the number of milligrams of CaCO3 equivalent hardness present per litre of water. Thus:
1 mg/L = 1 mg of CaCO3 eq hardness per L of water.
3. Clarke’s degree (oCl): Is number of grains (1/7000 lb) of CaCO3 equivalent hardness per gallon (10 lb) of water. Or it is parts of CaCO3equivalent hardness per 70,000 parts of water. Thus,
1oClarke = 1 grain of CaCO3 eq hardness per gallon of water.
4. Degree French (oFr): Is the parts of CaCO3 equivalent hardness per 105 parts of water. Thus,
1o Fr = 1 part of CaCO3 hardness eq per 105 parts of water.
Alkalinity: Alkalinity of sample can be estimated by titration with standard H2SO4 or HCI solution. Titration to pH 8.3 or decolourisation of phenolphthalein indicator will indicate complete neutralization of OH- and 1/2 of CO32-, while to pH 4.5 or sharp change from yellow to orange of methyl orange indicator will indicate total alkalinity.
To detect the different types of alkalinity, the water is tested for phenolphthalein and total alkalinity, using Equations:
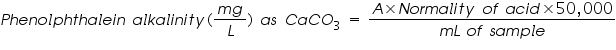
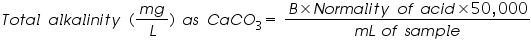
Where,
A = titrant (mL) used to titrate to pH 8.3
B = titrant (mL) used to titrate to pH 4.5
N = normality of the acid (0.02N H2SO4 for this alkalinity test)
50,000 = a conversion factor to change the normality into units of CaCO3
Once PA and TA are determined, then three types of alkalinities, i.e, hydroxides, carbonates and bicarbonates can be easily calculated from the table:
Result of titration | OH as alkalinity as CaCO3 | CO3 alkalinity as CaCO3 | HCO3 alkalinity as CaCO3 |
PA=0 | 0 | 0 | TA |
PA<1/2 TA | 0 | 2PA | TA-2PA |
PA=1/2 TA | 0 | 2PA | 0 |
PA>1/2TA | 2PA-TA | 2(TA-PA) | 0 |
PA=TA | TA | 0 | 0 |
Dissolved Oxygen:
Adequate dissolved oxygen concentrations are critical during all phases of striped bass and hybrid culture. Low dissolved oxygen concentrations can result in slower growth and induce the stress response predisposing the animals to infectious disease. Monitoring of dissolved oxygen concentrations is complicated by the rate at which they can change. In heavily stocked raceways, tanks, or flow-through systems, for example, an interruption of oxygenation may result in critically low dissolved oxygen concentrations within minutes due to consumption by the culture animals. Management of dissolved oxygen concentrations in ponds must also consider the daily rhythms of concentrations characteristic of ponds. Striped bass and its hybrids have different dissolved oxygen requirements at different stages in their lives. Striped bass also appear to require higher concentrations of dissolved oxygen relative to other temperate species. Generally, dissolved oxygen concentrations should be maintained as close to saturation as possible for best survival and growth.