UNIT 5
BUILDING MATERIALS
The raw material which are used for manufacturing of cement contains the following materials:
- Calcareous (chalk consist of limestone)
- Argillaceous (clay consist of silicates of alumina)
Wet Process manufacturing: On using the calcareous as a raw material then chalk must be crushed into minute particles and hence dispersed it in water in a wash mill. Wash mill used to break the solid materials into lumps/slurry. In similar wash mill clay is also broken up and mixed with water. Then this slurry is stored in the silo called as slurry silo where it is consistently stirred. The composition of raw material is checked again and if required corrected by adding clay or chalk materials as per requirement.
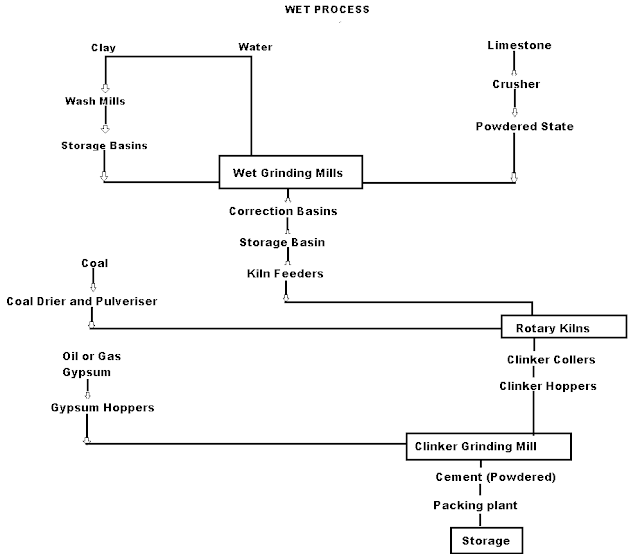
Cement Manufacturing Process Flow Chart
(i) Drying Zones : The raw material in slurry form is directly fed into the kiln which has more amount of water. It is the upper portion of the kiln. In this zone, water is evaporated at temperature 100-400°C.
(ii) Formations of modules: As the slurry gradually descends in the kiln, the carbon di oxide from slurry evaporates and small lumps formed which may be called as modules.
(iii) Burning Zone:- The modules enter in this zone where temperatures is kept about 1400-1500° C. The modules are converted into dark greenish balls and the product obtained in the kiln, known as clinker, is of varying size 5 to 20 mm. The clinkers are very hot when come out from this zone.
(iv) Cooling of Clinkers:- It is used for cool down the clinkers up to about 90°C.
Grinding: The cooled clinkers are finally ground in ball mills or tube mills.
Also, the gypsum is added during grinding about 2-4%. The gypsum acts as a retarder and so allows the cement to mix with sand or aggregate and to be placed in position. i.e. it increases the initial setting time of cement.
Storage and Packing: As cement comes out from grinding mills, it is collected in a hopper and taken in bucket elevator for storage in silos.
The cement from silos is packed by machines in bags. Each bag of cement contains 50 kg or 0.035 m3 of cement.
Dry Process manufacturing: This type of process for manufacturing the cement is available in the places where hard crystalline limestone and shales are available. The advantage of using this type of process is that fuel consumption is low.
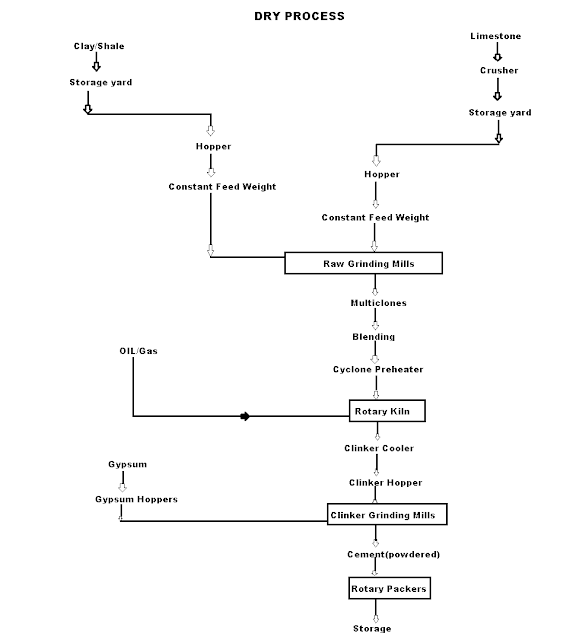
Mixing of Raw Materials: The used raw material undergo the following process:
- Crushing: The raw materials are broken in crushers to small fragments.
- Drying: The crushed materials are dried by heating at a sufficiently high temperature. It may be done in drying kilns.
- Reduction of size: The drying materials are then grind by using ball mills and tube mills to reduce the size of materials to find powder.
- Mixing in correct proportion: The finely dried materials are mixed in exact proportions. The mixing may be done either mechanically or by pneumatic methods.
Burning and Grinding: In this process, the raw materials mixed, fined and then fed into kiln whereas in the wet process, the raw materials are crushed separately and then directly mixed in correct proportion in the presence of water to make a fine thin paste known as Slurry.
On mixing up of cement with water to form plastic mass then it is called as cement paste. During hydration reaction, gel and crystalline products are formed. The inter-locking of the crystals binds the inert particles of the aggregates into a compact rock like material.
This process comprises of
(i) Setting
(ii) Hardening
Setting is defined as stiffening of the original plastic mass due to initial gel formation while hardening is development of strength, due to crystallization.
The hardening starts after setting as because of gradual progress of crystallization in the interior mass of cement. The strength developed by cement paste at any time depends upon the amount of gel formed and the extent of crystallization. The setting and hardening of cement is due to the formation of inter locking crystals reinforced by rigid gels formed by the hydration and hydrolysis of the constitutional compounds.
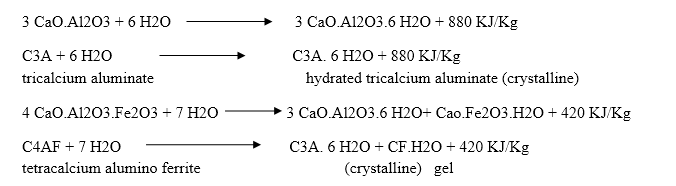
Dicalcium silicate also hydrolyses to tobermonite gel which contributes to initial setting.

Final setting and hardening of cement paste is due to the formation of tobermonite gel and crystallization of calcium hydroxide and hydrated tricalcium aluminate.
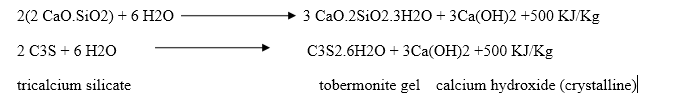
During setting and hardening of cement, some amount of heat is liberated due to hydration and hydrolysis reactions. The quantity of heat evolved during hydration of cement is 500 KJ/Kg.
Function of gypsum in cement :-
Tri calcium aluminate combines with water very rapidly.

After the initial setting, the paste becomes soft and the added gypsum retards the dissolution of tri calcium aluminate by forming insoluble calcium sulpho aluminate.
The cement additives enhance the quality of concrete for application in construction with special requirements. Concrete admixtures is defined as the material other than water aggregates and hydraulic cement and additives like slag and fiber reinforcement, used as an ingredient of concrete or mortar and added to the batch immediately before mixing to modify the properties of concrete in the plastic or hardened state. Some of the important purposes for which additives are used are:
(i) To increase the workability without increasing water content
(ii) To retard or accelerate time of initial setting
(iii) To reduce or prevent settlement
(iv) To improve pumpability
To reduce the rate of slump loss
Refractories: The different variety of material used in the creation of heat resisting containers i.e.; to be for the space afford for the evolution of gas in combustion process or for the holding up of molten charges. Refractories are inorganic, nonmetallic, porous, and heterogeneous. They are typically composed of oxides or non oxides like carbides, nitrides etc. of the following materials: silicon, aluminium, magnesium, calcium, and zirconium.
Refractories can be classified in multiple ways:
1) Based on Chemical composition
2) Fusion Temperature
3) Method Manufacture
4) Thermal Conductivity
Based on Chemical Composition:
Silica refractories: They consists of silicon oxide (SiO2) also known as silica. Silica refractories are produced from quartz or fused silica. Silica refractories contain at least 93 % SiO2. Various grades of silica brick have found extensive use in the iron and steel industry furnaces. In addition to high fusion point multi-type refractories, the other important properties of silica refractories are their high resistance to thermal shock and their high refractoriness. The outstanding property of silica brick is that it does not begin to soften under high loads until its fusion point is approached, they are slag resistive.
Fireclay refractories: Fireclay refractories comprise around 75 % of the production of refractories on a volume basis and are essentially hydrated aluminum silicates with minor proportions of other minerals. Typical composition consists of SiO2 less than 78 % and Al2O3 less than 44 %. As a type, they are extremely versatile and least costly of all refractory bricks and are extensively used in the iron and steel industry. Fireclay refractories are produced by firing of certain types of clays. The principal mineral for these refractories is kaolinite.
Fusion Temperature:
The phase transition of substance from solid to liquid is called as the Fusion Temperature. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which increases the substance's temperature to the melting point. At the melting point, the ordering of ions or molecules in the solid breaks down to a less ordered state, and the solid melts to become a liquid.
Refractory materials are classified into three types based on fusion temperature.
Normal refractories have a fusion temperature of 1580 to 1780 °C (e.g. Fire clay)
High refractories have a fusion temperature of 1780 to 2000 °C (e.g. Chromite)
Super refractories have a fusion temperature of > 2000 °C (e.g. Zirconia)
Method of manufacture:
The refractories can be manufactured by several methods consisting
(i) dry press process
(ii) fused cast process
(iii) hand molding process
(iv) forming process consisting of normal, fired or chemical bonded
(v) Unformed refractories such as monolithic, plastics, ramming masses, gunning materials etc. and are classified accordingly.
Thermal Conductivity:
Refractories may be classified by thermal conductivity as conducting, non-conducting, or insulating. E.g.: SiC and ZrC, whereas examples of non-conducting refractories are silica and alumina. Insulating refractories include calcium silicate materials, kaolin, and zirconia.
Insulating refractories are used to reduce the rate of heat loss through furnace walls. These refractories have low thermal conductivity due to a high degree of porosity, with a desired porous structure of small, uniform pores evenly distributed throughout the refractory brick in order to minimize thermal conductivity. Insulating refractories can be further classified into four types:
- Heat-resistant insulating materials with application temperatures ≤ 1100 ºC
- Refractory insulating materials with application temperatures ≤ 1400 ºC
- High refractory insulating materials with application temperatures ≤ 1700 ºC
- Ultra-high refractory insulating materials with application temperatures ≤ 2000 ºC
Ceramics: The word ceramic is borrowed from a Greek word “keramos” which means burnt stuff. Ceramics are inorganic non-metallic material that are processed and used at high temperatures. They include silicates, metallic oxides and their combination ceramic materials having wide range of properties are produced for different applications.
Ceramics generally consist of three major components:
Plastic portion: This is usually provided by clay, which imparts the necessary plasticity and workability.
A flux or glassy materials: This is provided by (kAlSio8), which helps in bonding and cementing the ingredients together.
A non-plastic refractory crystalline portion: This is provided by silica, which contribute mechanical strength.
Ceramics are mainly classified into three types:
1. Clay products
2. Refractories
3. Glasses according to their characteristic features.
Clay products: Clay products are divided into three main types:
Structural clay products which contain iron oxides. They are used for bricks, tiles and similar products.
White wares which are pales substances such as porcelain and chain.
Chemical stone wares which are specially treated to be hard, and non-porous.
Properties of ceramics:
- They are usually hard and brittle in nature and generally being in the form of amorphous or glassy solids.
- The atomic bonding in these materials is of mixed ionic or covalent character.
- They have good electrical resistance and act as insulator.
- They have high temperature resistance.
- They have good resistance to chemical attack and weathering.
- They have high compressive strength and textile strength.
- The ceramic materials have lower spalling resistance than metals.
- They are resistance towards corrosion.
- They have high hardness values.
- They are brittle in nature.
- Ceramic materials are good thermal and electrical insulators this is due to the absence of conduction electrons.
- Ceramic materials are polarizable.
- Some ceramic materials can be highly polarized with electric charge. They are used as dielectric materials for capacitors.
Applications of ceramics:
Ceramic materials are not amenable to a great range of processing.
Non-crystalline ceramics, being glasses, tend to be formed from melts. The glass is shaped when it is fully molten.
They are used in Automotive.
They are used in electronic applications.
They are used in Aerospace.
They are high wear resistive, temperature resistive and a high level of corrosion resistive.
They are used in cutting tools and mechanical pumps.