Module 1
Atomic and Molecular Structure
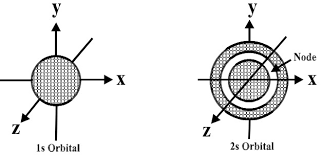
Shape of s orbital:
The s orbital is spherical having the nucleus at its centre which in two dimensions and can be seen as a circle. The s-orbitals are spherically symmetric having the probability of finding the electron at a given distance equal in all the directions. The size of the s orbital is also found to increase with the increase in the value of the principal quantum number, thus, 4s > 3s> 2s > 1s.
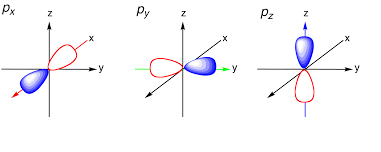
Shape of p orbital:
Each p orbital consists of two sections that is known as lobes which lie on either side of the plane passing through the center that is nucleus. The three p orbitals differ in the way lobes are oriented whereas they are identical in terms of size shape and energy. The lobes lie along one of the x, y or z-axis, these three orbitals are given the designations 2px, 2py, and 2pz. Thus, we can say that there are three p orbitals whose axes are mutually perpendicular. Similar to s orbitals, size, and energy of p orbitals increases with an increase in the principal quantum number (4p > 3p > 2p).
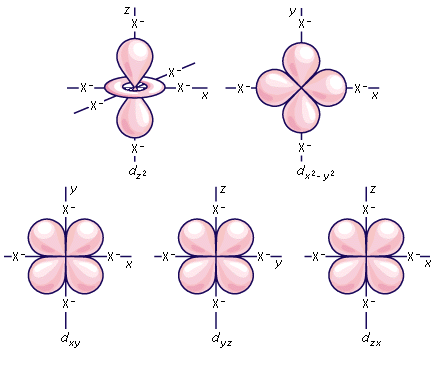
Shape of d orbital:
The magnetic orbital quantum number for d orbitals is given as (-2,-1,0, 1,2). Hence, we can say that there are five d-orbitals. These orbitals are designated as dxy, dyz, dxz, dx2–y 2 and dz2. Out of these five d orbitals, shapes of the first four d-orbitals are similar to each other, which is different from the dz2 orbital whereas the energy of all five d orbitals is the same.
The distribution of electrons of an atom or molecule in atomic or molecular orbitals is called as the electronic configuration. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Electronic configuration describe the specific location of each electrons in atoms or molecules.

The electron distribution over various energy level is governed by the following rules –
The maximum number of electrons in any main energy level (shell) is given by, ‘2n2’, where, n is an integer and representing the “principal quantum number”. For different main energy levels the value of ‘n’ and maximum number of electrons are given in table below-
Sl. No. | Energy level or Orbit (shell) | Principal quantum number ‘n’ | Maximum Number of electrons (2n2) |
1 | K | 1 | 2×12 = 2 |
2 | L | 2 | 2×22 = 8 |
3 | M | 3 | 2×32 = 18 |
4 | N | 4 | 2×42 = 32 |
The each main shell (energy level) is subdivided into sub shells. These sub shell are called orbitals. These sub shells /orbitals are designated by s, p, d, f etc. with corresponding orbital quantum number, l = 0, 1, 2, 3, 4,…..(n-1) etc. The number of sub shells in any main shell is equal to “principal quantum number” ‘n’.
Atomic Orbitals is the mathematical function which is responsible for the determination of location and wave like behavior of an electron in the atom. Atomic orbital is the region where the electrons are present in the atoms. Hence the orbitals in an atom is identified from their unique values i.e.; n, l, m.
Properties:
- Electron orbits the nucleus as a standing waves.
- The electron is at the random position means they never stay at a single position.
Molecular Orbital is the mathematical function which is responsible for the determination of location and wave like behavior of an electron in the molecule. In molecular orbital, the electrons are allowed to interact with more than one atomic nucleus at a time.
Linear Combination of Atomic Orbital (LCAO):
The approximate method used to represent molecular orbital is called as the Linear Combination of Atomic Orbital. It is a quantum superposition of atomic orbital and a technique for calculating molecular orbital in quantum chemistry.
Rules for the Linear Combination of Atomic Orbital are:-
- The combining atoms should have the same symmetry along the molecular axis for proper combination. e.g. All the sub-orbitals of 2p have same energy but still, the 2pz orbital of an atom can only combine with a 2pz orbital of another atom but cannot combine with 2px and 2py orbital as they have a different axis of symmetry.
- The two atomic orbital will combine to form molecular orbital. Greater is the extend of overlap of atomic orbital; greater will be the nuclear density.
The combining atomic orbital must be of equal energy or approximately same energy.
Bonding Molecular Orbitals
When addition of wave function takes place, the type of molecular orbitals formed are called Bonding Molecular orbitals and is represented by
ΨMO = ΨA + ΨB.
They have lower energy than atomic orbitals involved. It is similar to constructive interference occurring in phase because of which electron probability density increases resulting in formation of bonding orbital. Molecular orbital formed by addition of overlapping of two s orbitals. It is represented by s.
Anti-Bonding Molecular Orbitals
When molecular orbital is formed by subtraction of wave function, the type of molecular orbitals formed are called Antibonding Molecular Orbitals and is represented by
ΨMO = ΨA - ΨB.
They have higher energy than atomic orbitals. It is similar to destructive interference occurring out of phase resulting in formation of antibonding orbitals. Molecular Orbital formed by subtraction of overlapping of two s orbitals. It is represented by s* (*) is used to represent antibonding molecular orbital) called Sigma Antibonding. Therefore, Combination of two atomic orbitals results in formation of two molecular orbitals, bonding molecular orbital (BMO) whereas other is anti-bonding molecular orbital (ABMO).
BMO has lower energy and hence greater stability than ABMO. First BMO are filled then ABMO starts filling because BMO has lower energy than that of ABMO.
Formation of molecular orbitals occurs by the combination of atomic orbitals of proportional symmetry and comparable energy. Therefore, a molecular orbital is polycentric and atomic orbital is monocentric. Number of molecular orbitals formed is equal to the number of atomic orbitals.
Certain rules are to be followed while filling up molecular orbitals with electrons in order to write correct molecular configurations:
Aufbau Principle – This principle states that those molecular orbital which have the lowest energy are filled first.
Pauli’s Exclusion Principle – According to this principle each molecular orbital can accommodate maximum of two electrons having opposite spins.
Hund’s Rule – This rule states that in two molecular orbitals of the same energy, the pairing of electrons will occur when each orbital of same energy consist one electron.
Energy Level Diagram:
The factors upon which relative energies of molecular orbitals depend are:
(i) Energies of the Atomic orbitals combining to form Molecular Orbitals.
(ii) The extent of overlapping between the atomic orbitals. The greater the overlap, the more the bonding orbital is lowered and the anti-bonding orbital is raised in energy relative to AOs
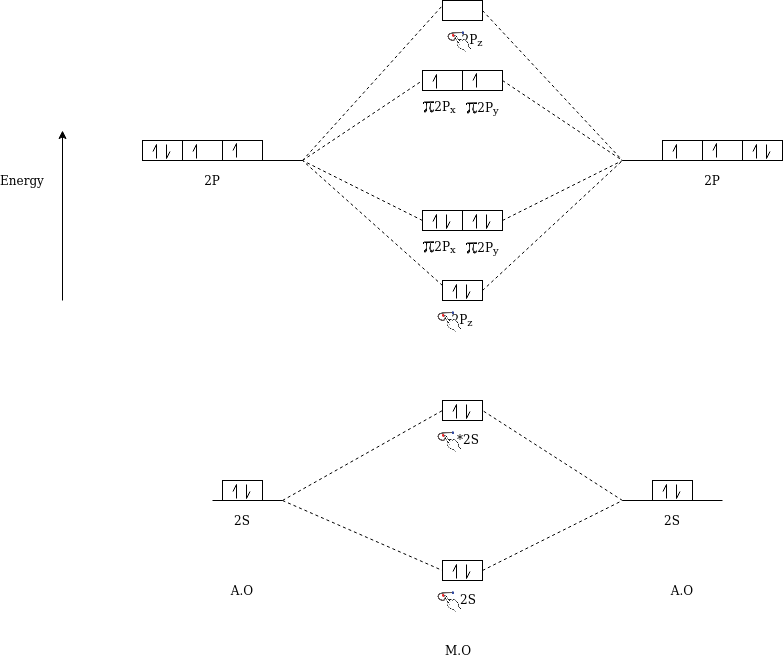
1s Atomic Orbitals (AOs) of two atoms form two Molecular Orbitals (MOs) designated as s1s and s *1s.The 2s and 2p orbitals (eight AOs of two atoms) form four bonding MOs and four anti-bonding MOs as:
Bonding MOs: σ 2s, σ 2pz, π 2px, π 2py
Anti – Bonding MOσ: σ *2s, σ *2pz, π *2px, π *2py
Using Spectroscopy, the energy levels of these molecular orbitals are determined experimentally. The order of increasing energy of molecular orbitals obtained by combination of 1s, 2s and 2p orbitals of two atoms is →
σ1s, σ *1s, σ 2s, σ *2s, σ 2pz, π 2px = π 2py, π *2px= π *2py, σ *2pz
Bonding Molecular Orbitals
When addition of wave function takes place, the type of molecular orbitals formed are called Bonding Molecular orbitals and is represented by
ΨMO = ΨA + ΨB.
They have lower energy than atomic orbitals involved. It is similar to constructive interference occurring in phase because of which electron probability density increases resulting in formation of bonding orbital. Molecular orbital formed by addition of overlapping of two s orbitals. It is represented by s.
Anti-Bonding Molecular Orbitals
When molecular orbital is formed by subtraction of wave function, the type of molecular orbitals formed are called Antibonding Molecular Orbitals and is represented by
ΨMO = ΨA - ΨB.
They have higher energy than atomic orbitals. It is similar to destructive interference occurring out of phase resulting in formation of antibonding orbitals. Molecular Orbital formed by subtraction of overlapping of two s orbitals. It is represented by s* (*) is used to represent antibonding molecular orbital) called Sigma Antibonding. Therefore, Combination of two atomic orbitals results in formation of two molecular orbitals, bonding molecular orbital (BMO) whereas other is anti-bonding molecular orbital (ABMO).
BMO has lower energy and hence greater stability than ABMO. First BMO are filled then ABMO starts filling because BMO has lower energy than that of ABMO.
Formation of molecular orbitals occurs by the combination of atomic orbitals of proportional symmetry and comparable energy. Therefore, a molecular orbital is polycentric and atomic orbital is monocentric. Number of molecular orbitals formed is equal to the number of atomic orbitals.
Be2
Be2 Electronic Configuration :- 1S2 2S1
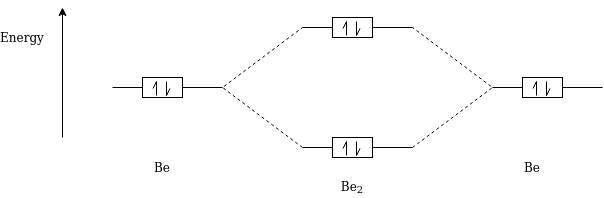
Magnetic Property : Diamagnetic in Nature
Bond Order :- 1/2(Nb-Na)
= 1/2(2-2)
=0
O2
Bond Order= ½ [Nb-Na]
=1/2 [8-4]
=2
Magnetic Property= Paramagnetic Character
CO
