MODULE 3
Intermolecular Forces & Critical Phenomena
The properties like melting point and boiling point can be a measure to show how strong the attractive forces exist between molecules are individual molecules. We can also call these as intermolecular forces. The general principle being as bons become more polarized, the charges on the atoms become greater, subsequently the intermolecular attraction increases, which leads to higher boiling point.
The three intermolecular forces are as follows
- Ionic bonds
- Hydrogen bonding called
- Van der Waals dipole-dipole interaction.
- Ionic forces:
Are the interactions between charged molecules or atoms (ions).
Positively charged ions, are Na (+), Li (+), and Ca (+), they are called as Cations, on the other hand the negatively charged ions are Cl (-), Br (-), Ho (-) they are Anions. The attractive forces between ions that are oppositely charged is described under the Coulomb’s Law, as the law says the force increases with charge and decreases as the distance between these ions increases. The highly polarised or charged nature of the ionic molecules is reflected in the nature of their melting point. For e.g. NaCl has a melting point of 801oc. The ionic molecules also show the property of high solubility in water.
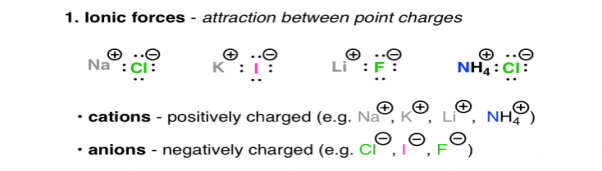
Fig. 1: Ionic forces – attraction between point charges
- Hydrogen bonding:
Hydrogen bonding occurs in molecules that are highly electromagnetic in nature, elements like F, O or N are directly bound to hydrogen. As hydrogen has an electronegativity of 2.2, they are not as polarized as ionic bonds and have some covalent character. But still the hydrogen bond is polarized and possess a dipole.
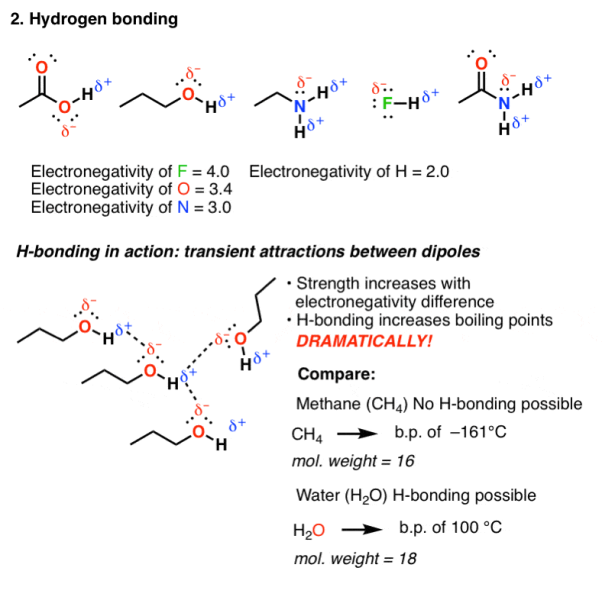
Fig. 2: Hydrogen bonding
Hydrogen bonding is an attractive interaction that occurs when the dipole of one molecule can align with the dipole from another molecule. Since the molecular motion of these molecules are rapid in a solution, these bonds are short lived therefore HO and NH are bonding molecules containing these functional groups that tend to have high boiling point.
- Van der Waals dipole- dipole interaction
Other groups other than hydrogen can be involved in covalent bonding with strong electronegative atoms. For example, in the figure given below each of the molecules contain a dipole:
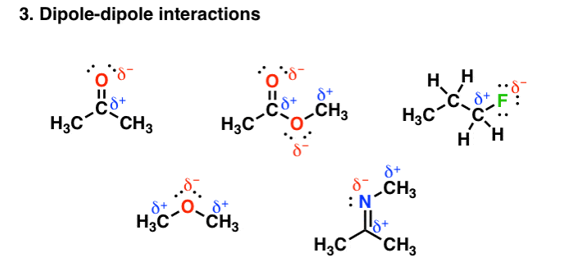
Fig. 3: Dipole-dipole interaction
- Dipoles are created by differences in electronegativities between nearby atoms
- Interactions between opposite-charged dipoles are attractive
- Similar in origin to hydrogen bonding, but since the differences in electronegativities are smaller
- Carbon is more electronegative (2.5) than hydrogen (2.2): the magnitudes of the dipoles are smaller, and these interactions are weaker.
These dipoles interact with each other in a very unique and attractive manner and thereby increase the boiling point. But the main aspect is the bonding depends on the electronegativity differences. The electronegativity of carbon =2.5, but that of Oxygen and Nitrogen is less than hydrogen whose electronegativity is=2.2, therefore the polar interaction is not so very strong. So, on an average these forces tend to be weaker than in hydrogen bonding
To sum up, boiling points are a measure of intermolecular forces and boiling point increases with molecular weight and surface area. The intermolecular forces increase with the increase in polarization of bonds.
In a real gas the molecular volume is not negligible, also cohesive or repulsive intermolecular forces mean that the pressure applied on the containing vessel is less than that of an Ideal gas. Therefore, the equation of state needs that the pressure p is increased by a quantity proportional to the density or, by the quantity inversely proportional to the volume. The van der Waals equation has played an important role in describing fluids, i.e. both liquids and gases.
Van der Waals equation is also known as Van der Waals equation of state for real gases and is not applicable or follows ideal gas law. According to the state of ideal gas law, PV = nRT where P is the pressure, V is the volume, n is the number of moles, T is the temperature and R is the universal gas constant. The Van der Waals Equation derivation is explained below.
Derivation of Van der Waals equation
For the state of real gas, using Van der Waals equation, the volume of a real gas is given as (Vm – b), where b is volume occupied by per mole.
Therefore, ideal gas law when substituted with V = Vm – b is given as:
P(Vm−b) =nRT
Because of intermolecular attraction P was modified as below
(P+a/Vm2) (Vm−b) =RT
(P+an2/V2) (V−nb) =nRT
Where,
Vm: molar volume of the gas
R: universal gas constant
T: temperature
P: pressure
V: volume
Thus, Van der Waals equation can be reduced to ideal gas law as PVm = RT.