Module 6
Water
Hardness can be defined as a soap consuming capacity of water sample. Soaps are sodium salts of fatty acids like oleic acid, palmetic acid and stearic acid. They dissolve readily .in water to form lather due to which it has cleansing property.


- But compounds of fatty acids with other metals done dissolve in water.
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd.
 2
2
(calcium stearate)
- If water contains other metal ions like calcium and magnesium ions they react with sodium salts of long chain fatty acids to form in soluble soap which we observe as curd
- These other metal ions are responsible for the hardness of water most important metal of ions which cause hardness to water are calcium and magnesium ions.
- The hardness of water along can be calculated from the amount of calcium and magnesium ions present in water along with bicarbonates, sulphates chlorides and nitrates.
TYPES OF WATER | HARDNESS |
Soft | 0 – 75 |
Moderately hard | 75 – 150 |
Hard | 150 – 300 |
Very hard | Above 300 |
1. Parts Per Million (ppm):- is the parts of CaCO3 equivalent hardness per 106 parts of water i.e., 1ppm= 1 part of CaCO3 equivalent hardness in 106 parts of water.
2. Milligrams Per Litre (mg/L):- number of milligrams of CaCO3 equivalent hardness present per liter of water.
1mg/L=1mg of CaCO3
Equivalent hardness of 1L of water= 1kg=1000g=106mg.
∴1mg/L=1mg of CaCO3 eq per 106 mg of water=1ppm.
3. Clarke’s degree (0Cl):- the no. Of grains (1/7000lb) of CaCO3 equivalent hardness per gallon (10lb) of water or it is parts of CaCO3 equivalent hardness per 70,000 parts of water.
∴1 0Cl= 1 grain of CaCO3 eq hardness per gallon of water
= 1 part of CaCO3 hardness eq per 105 parts of water.
4. Degree French ( o Fr):- parts of CaCO3 equivalent hardness per 105 parts of water.
∴1 0 Fr= 1 part of CaCO3 equivalent hardness per 105 parts of water.
5. Milli-equivalent per liter (meq/L):- is the number of milli-equivalents of hardness present per liter.
1meq/L = 1meq of CaCO3 per liter of water
= 10-3 x 50 g of CaCO3 eq. Per liter
= 50 mg of CaCO3 eq. Per liter
= 50 mg/L of CaCO3 eq.
= 50ppm.
Types of hardness: -
- Temporary hardness (carbonate) :-
- When water containing calcium and magnesium bicarbonates is heated, bicarbonate decompose and from insoluble carbonate and form hydroxide.
- On filtering such water, soft water is obtained.
- The hardness which can be removed by more boiling is referred as ‘temporary hardness ’ or bicarbonate hardness.

 Ca
Ca

Mg 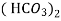
 Mg
Mg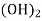 + 2 CO
+ 2 CO 
(Bicarbonates)
II. Permanent hardness :-
- The term permanent hardness ornon carbonate is the term applied to the hardness caused by dissolved chlorides , nitrates and sulphate of calcium and magnesium.
- This cannot be removed by boiling the water sample.
- Sum of temporary and permanent hardness is referred to as total hardness.
III. Alkaline or carbonate and non – alkaline or non – carbonate hardness :-
- Like all carbonate and bicarbonate , calcium and magnesium carbonate and bicarbonate are alkaline.
- Then hardness due to the carbonate and bicarbonate is called alkaline hardness or carbonate hardness.
- The alkalinity can be measures by titration with standard mineral acid using methyl orange and or phenol phthalein as an indicator.
4. As the sulphate and chloride are neutral salts , the hardness caused by presence of calcium and magnesium sulphate , chlorides and nitrates is termed as non alkaline hardness or non carbonate hardness.
IV. Total hardness :-
The hardness due to all hardness causing salts , known as total hardness.
Total hardness = temporary + permanent
Estimation of hardness :-
Hardness of weather can be determined by two methods.
1) Soap solution method :-
- Total hardness of water can be determined by titrating a fixed volume of water sample (100ml) against standard alcoholic soap solution.
- Appearance of stable lather which persists for two minutes is the end point of titration.
- In the beginning sodium soap will precipitate all the hardness causing metal ions in the form of their soap (card) and then it will form free lather.
- If same water sample is boiled for 30minutes and then titrated against same soap solution the titration reading corresponds to permanent hardness.
The difference between two measurements corresponds to the temporary hardness of water.
Estimation of hardness of water by complexometric method:
Complexometric titration is a form of volumetric analysis in which the formation of a colored complex is used to indicate the end point of a titration. Complexometric titrations are particularly useful for the determination of a mixture of different metal ions in solution. An indicator capable of producing an unambiguous color change is used to detect the end-point of the titration. Complexometric titration are those reactions where a simple ion is transformed into a complex ion and the equivalence point is determined by using metal indicators or electrometrically.
- The reaction reaches equilibrium rapidly after each portion of titration is added.
- Interfering situations do not arise.
- A complexometric indicator capable of locating equivalence point with fair accuracy is available.
Titration with EDTA
EDTA(Ethylene Diamine Tetra Acetic Acid) , has four carboxyl groups and two amine groups that can act as electron pair donors. The ability of EDTA to potentially donate its six lone pairs of electrons for the formation of coordinate covalent bonds to metal cations makes EDTA a hexadentate ligand. However, in practice EDTA is usually only partially ionized, and thus forms fewer than six coordinate covalent bonds with metal cations.
Disodium EDTA is commonly used to standardize aqueous solutions of transition metal cations. Disodium EDTA (often written as Na2H2Y) only forms four coordinate covalent bonds to metal cations at pH values ≤ 12. In this pH range, the amine groups remain protonated and thus unable to donate electrons to the formation of coordinate covalent bonds. Note that the shorthand form Na4−xHxY can be used to represent any species of EDTA, with x designating the number of acidic protons bonded to the EDTA molecule.
EDTA forms an octahedral complex with most 2+ metal cations, M2+, in aqueous solution. The main reason that EDTA is used so extensively in the standardization of metal cation solutions is that the formation constant for most metal cation-EDTA complexes is very high, meaning that the equilibrium for the reaction:
M2+ + H4Y → MH2Y + 2H+
Lies far to the right. Carrying out the reaction in a basic buffer solution removes H+ as it is formed, which also favors the formation of the EDTA-metal cation complex reaction product. For most purposes it can be considered that the formation of the metal cation-EDTA complex goes to completion, and this is chiefly why EDTA is used in titrations and standardizations of this type.
Principle: This is a complex metric method. It is in the form of its sodium salt which yields the anion and this forms complex with Ca+2 and Mg+2 ions.
(Molecular Wt. - 372.24, Equivalent Wt. - 186.14 i.e., M=2N)
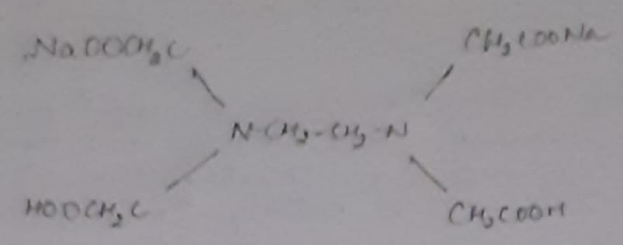
In order to determine the equivalence point (i.e., just completion of metal-EDTA complex formation) indicator Eriochrome Black-T (EBT) an alcoholic solution of blue dye is employed which forms an unstable wine red complex with Ca+2 and Mg+2 ions. The indicator is effective at about pH 10. When EBT is added to hard water, buffered to a pH of about 10 (employing NH4OH-NH4Cl buffer), a wine red unstable complex is formed. Thus,
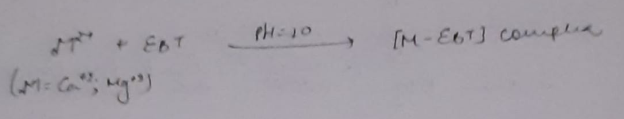
During the course of titration against EDTA solution, EDTA combines with M+2 (or Ca+2 or Mg+2) ions from stable complex M-EDTA and releasing free EBT, which instantaneously combines with M+2 ions still present in the solution, thereby wine red color is retained. Thus, titration

When nearly all M+2 (Ca+2 or Mg+2) ions have formed [M-EDTA] complex, then next drop of EDTA added drop wise displace the EBT indicator from [M-EBT] complex and wine red color changes to blue color (due to EBT). Thus at equivalence point

Steps involved:
1. Preparation of Standard Hard Water: Dissolve 1gm of pure dry CaCO3 in minimum quantity of dil. HCl and then evaporate the solution to dryness on water bath. Dissolve the residue n distilled water to make 1L solution. Each 1ml of this solution contains 1mg of CaCO3 hardness. 6
2. Standardization of EDTA solution: Rinse and fill the burette with EDTA solution. Pipette out 50ml of standard hard water in a conical flask. Add 10-15ml of buffer solution and 4 drops of indicator. Titrate with EDTA solution till wine red color changes to clear blue. Let the volume used be V1ml.
3. Titration of Unknown Hard Water: Titrate 50ml of water sample just in step5. Let the volume used be V2ml.
4. Titration of Permanent Hardness: Take 250ml of water sample in a large beaker. Boil till the volume is reduced to about 50ml (all the bicarbonates are decomposed into insoluble CaCO3+Mg(OH)2). Filter, wash the precipitate with distilled water collecting filtrate and washings in a 250 ml measuring flask. Finally make up the volume to 250ml with distilled water.
Ion exchange or de ionization or de mineralization ion exchange resins are insoluble cross linked long chain organic polymers with a microporous structure and the functional group attached to the chains are responsible for the ion exchange properties resins contaning acidic function groups are capable of exchaning their anions with other anions which comes in their contact the ion exchange resins may be classified as :-
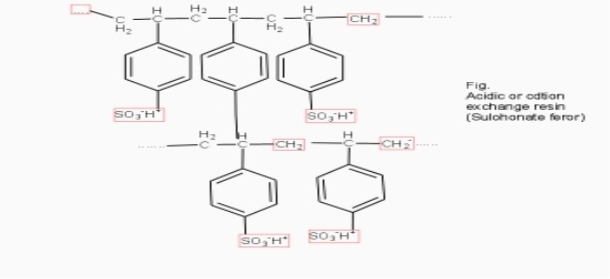
Cation exchange resins ( RH+) :-
They are mainly styrene divinly benzene co-polymers which on sulphonation or carbonoxylation become capable to exchange their hydrogen ions with the cations in the water.
- Anion exchange resins :-
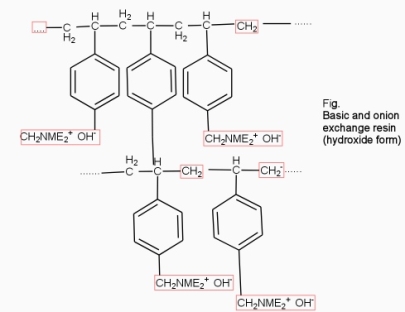
They are styrene – divinly benzene or amine fermaldehyde which contain amino or quaternary ammonium or4 quaternary phosphonium or tertiary sulphonium group as an integral part of the resin matrix.these after treatment with dilute NaOH solution become capable to exchange their OH- anions with anions in water .
Processes :-
The hard water is passed first through cation exchange column which removes all the cations like Ca²+ , Mg² + e.t.c . From it and equivalent amount of H+ ions are released from this column Water
2RH+ + Ca².+ ---------------------- R2Ca2+ + 2H
2RH+ Mg2+ --------------------------- R2Mg2+ 2H+
After cation exchange column the hard water passed through anion like cl- . Present in the water and equivalent amount of OH- ions are released from this column to water,

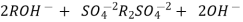
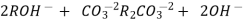
H+ And OH – ions ( released from cation exchange and anion exchange columns respectively ) get combined to produce water molecule.
H+ + OH -  H2O
H2O
Thus,
The water coming out form the exchanger is free from cations as well as an ions . Ion free water is known as de mineralizes water.
Regeneration :-
When capacities of cation and anion exchangers to exchange H+ and OH- ions respectively . Are lost they are then said to be exhausted.
The exhausted cation exchange column is regenerated by passing a solution of dil.HclH2SO4. The regeneration can be represented as


 ( washing )
( washing )
The column is washed with deionized water and washing ( which contains Ca2+ and Cl2- ions ) is passed to sink or drain.
The exhausted anion exchange column is regenerated by passing a solution of dil. NaOH.
The column is washed with de-ionized water and washing (which contains NA+ and 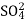 ions ) is pass to sink or drain.
ions ) is pass to sink or drain.
The regenerated ion exchange ion exchange resins are then used again.
Advantages :-
- The processes can be used to soften highly acidic or alkaline waters.
- It produces water of very low hardness ( say 2 ppm ) so it is very good for treating water for use in high pressure boilers.
Disadvantages :-
- The equipment is costly and more expensive chemicals are needed.
- If water contains turbidity, then the out- put of the processes is reduced .the turbidity must be below 10 ppm if it is more it has to be removed first by filtration.
Biochemical Oxygen Demand:
The determination of the Biochemical Oxygen Demand or Biological Oxygen Demand (BOD) evaluates the amount of biodegradable organic material present in wastewater, effluent and polluted waters. The BOD test reflects the amount of dissolved oxygen (DO) consumed by bacteria while oxidizing these materials. Dissolved oxygen is essential for the life of aquatic fauna and flora, and the BOD test is a measure of the ecological impact that effluent water may have on the receiving body of water (river, lake, etc.). This test is often required in discharge permits, as it is a means of assessing the degree of water pollution.
Two methods are widely used for BOD measurement: the dilution method and the manometric method:
- Dilution method
This is a standard method. As an example, the APHA standard methods 5210B from the American Public Health Association describes in detail this protocol. Dilution water is prepared by adding inorganic nutrients and buffer salts to purified water. If the sample does not contain sufficient amounts of bacteria, or contains compounds toxic to bacteria (e.g. Chlorinated effluent), it may be necessary to add microbial seed as well. Various dilution levels of the sample water are then prepared using the dilution water. The BOD bottles are filled to the top, capped and sealed. They are incubated in the dark at 20°C for 5 days. The levels of dissolved oxygen are measured prior to and after the 5-day incubation period. The difference between these two values, corrected for the dilution and the blank, is the BOD5 value. BOD tests results are expressed in mg/L of dissolved oxygen. - Manometric method
In this test, a manometer is fitted into a bottle containing the undiluted sample. It continuously measures the drop in air pressure in the bottle, which reflects the amount of oxygen uptake by the sample. This method is easier than the dilution method because no dilution is necessary, and continuous measurements are obtained.
The presence of toxicants or poor seeding material in water samples may lead to falsely low BOD results. Therefore, it is recommended to regularly use a glucose - glutamic acid (GGA) solution as a standard check solution. The oxygen uptake of this solution should be 198 +/- 30.5 mg/L.
Chemical Oxygen Demand: The chemical oxygen demand (COD) is a measure of water and wastewater quality. The COD test is often used to monitor water treatment plant efficiency. This test is based on the fact that a strong oxidizing agent, under acidic conditions, can fully oxidize almost any organic compound to carbon dioxide. The COD is the amount of oxygen consumed to chemically oxidize organic water contaminants to inorganic end products.
The COD is often measured using a strong oxidant (e.g. Potassium dichromate, potassium iodate, potassium permanganate) under acidic conditions. A known excess amount of the oxidant is added to the sample. Once oxidation is complete, the concentration of organics in the sample is calculated by measuring the amount of oxidant remaining in the solution. This is usually done by titration, using an indicator solution. COD is expressed in mg/L, which indicates the mass of oxygen consumed per liter of solution.
COD is a second method of estimating how much oxygen would be depleted from a body of receiving water as a result of bacterial action. The COD test uses a strong chemical oxidizing agent (potassium dichromate or potassium permanganate) to chemically oxidize the organic material in the sample of wastewater under conditions of heat and strong acid. The COD test has the advantage of not being subject to interference from toxic materials, as well as requiring only two or three hours for test completion, as opposed to five days for the BOD test. It has the disadvantage of being completely artificial, but is nevertheless considered to yield a result that may be used as the basis upon which to calculate a reasonably accurate and reproducible estimate of the oxygen-demanding properties of a wastewater. The COD test is often used in conjunction with the BOD test to estimate the amount of non-biodegradable organic material in a wastewater. In the case of biodegradable organics, the COD is normally in the range of 1.3 to 1.5 times the BOD. When the result of a COD test is more than twice that of the BOD test, there is good reason to suspect that a significant portion of the organic material in the sample is not biodegradable by ordinary microorganisms. As a side note, it is important to be aware that the sample vial resulting from a COD test can contain leachable mercury above regulatory limits. If such is the case, the sample must be managed as a toxic hazardous waste.
Electrodialysis (ED) is used to transport salt ions from one solution through ion-exchange membranes to another solution under the influence of an applied electric potential difference. This is done in a configuration called an electrodialysis cell. The cell consists of a feed (dilute) compartment and a concentrate (brine) compartment formed by an anion exchange membrane and a cation exchange membrane placed between two electrodes. In almost all practical electrodialysis processes, multiple electrodialysis cells are arranged into a configuration called an electrodialysis stack, with alternating anion and cation exchange membranes forming the multiple electrodialysis cells. Electrodialysis processes are different from distillation techniques and other membrane based processes (such as reverse osmosis (RO)) in that dissolved species are moved away from the feed stream rather than the reverse. Because the quantity of dissolved species in the feed stream is far less than that of the fluid, electrodialysis offers the practical advantage of much higher feed recovery in many applications.
Treatment processes for the production of freshwater from any kind of water sources such as groundwater and surface water, as well as recycled process and wastewater, are becoming more and more important to cope with rapidly increasing water demand. Increased nitrate concentrations, mainly caused by non-clarified wastewater or excessive application of artificial fertilizer and manure in agriculture, are found quite often in groundwater. Also many surface waterworks are confronted with the necessity to remove dissolved ionic substances. Common treatment methods used at waterworks are a combination of chemical oxidation, coagulation-flocculation, sand filtration, and disinfection. However, in recent years, membrane technology has become an extraordinarily useful tool for the production of freshwater.
Applications:
- Large scale brackish and seawater desalination and salt production.
- Small and medium scale drinking water production (e.g., towns & villages, construction & military camps, nitrate reduction, hotels & hospitals)
- Water reuse
- Pre-demineralization
- Food processing
- Agricultural water
- Glycol desalting
- Glycerin purification
Reverse Osmosis:
Reverse osmosis is a process where the water is separated from the salts in the source water by pressure-driven transport through a membrane. This process uses semi-permeable membrane and applied pressure to preferentially induce water permeation through the membrane while rejecting salts. The RO plant uses less energy than thermal desalination process. This process uses thin-film composite membrane that too comprises of ultra thin aromatic polyamide thin film. The used polyamide film gives the transparent properties while the remaining part provides the mechanical supports. The polyamide films are very dense void free polymer with high surface area allowing for its high permeability. RO desalination plant include source water intake system, pretreatment facilities and high pressure feed pumps, RO membrane trains, energy recovery and desalinated water conditioning system. The intake system may be the open surface water intake or series of seawater beach wells. The pretreatment system may be the screening, chemical conditioning, sedimentation or filtration that totally depends on the used quality water further the filtered water is conveyed by transfer pump from filtrate water storage tank through cartridge filter and into the suction pipe of high pressure RO feed pumps. The cartridge filters are designed in such a manner that can retain 1 to 20 microns particles which remained in the source water after pretreatment. The high pressure feed pumps are designed to deliver the source water to the RO membranes at pressure required for membrane separation of the fresh water from the salts. The actual required feed pressure is site-specific and is mainly determined by the source water salinity and the configuration of the RO system.
Ultra filtration:
Ultrafiltration (UF) is a membrane filtration process similar to Reverse Osmosis, using hydrostatic pressure to force water through a semi-permeable membrane. The pore size of the ultrafiltration membrane is usually 103 - 106 Daltons. Ultrafiltration (UF) is a pressure-driven barrier to suspended solids, bacteria, viruses, endotoxins and other pathogens to produce water with very high purity and low silt density.
Ultrafiltration (UF) is a variety of membrane filtration in which hydrostatic pressure forces a liquid against a semi permeable membrane. Suspended solids and solutes of high molecular weight are retained, while water and low molecular weight solutes pass through the membrane. Ultrafiltration is not fundamentally different from reverse osmosis, microfiltration or nanofiltration, except in terms of the size of the molecules it retains.
A membrane or, more properly, a semi permeable membrane, is a thin layer of material capable of separating substances when a driving force is applied across the membrane. Once considered a viable technology only for desalination, membrane processes are increasingly employed for removal of bacteria and other microorganisms, particulate material, and natural organic material, which can impart color, tastes, and odors to the water and react with disinfectants to form disinfection byproducts (DBP).
As advancements are made in membrane production and module design, capital and operating costs continue to decline. The pressure-driven membrane processes discussed in this fact sheet are microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), and reverse osmosis (RO).
Benefits
- No need for chemicals (coagulants, flocculates, disinfectants, pH adjustment)
- Size-exclusion filtration as opposed to media depth filtration
- Good and constant quality of the treated water in terms of particle and microbial removal
- Process and plant compactness
- Simple automation
- Environment friendly