Unit - 2
Stereochemistry
Stereochemistry deals with three-dimensional representation of molecule in space. This has sweeping implications in biological systems. For example, most drugs are often composed of a single stereoisomer of a compound. Among stereoisomers one may have positive effects on the body and another stereoisomer may not or could even be toxic.
The study of stereochemistry focuses on stereoisomers and spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes method for determining and describing these relationships; the effect on the physical or biological properties
The wedge and dash representations of stereochemistry can often become cumbersome, especially for large molecules which contain a number of stereocenters. An alternative way to represent stereochemistry is the Fischer Projection, which was first used by the German chemist Emil Fischer. The Fischer Projection represents every stereocenter as a cross. The horizontal line represents bonds extending out of the plane of the page, whereas the vertical line represents bonds extending into the plane of the page.
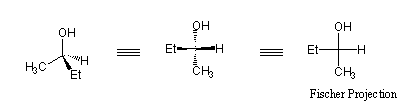
Manipulations of Fischer Projections
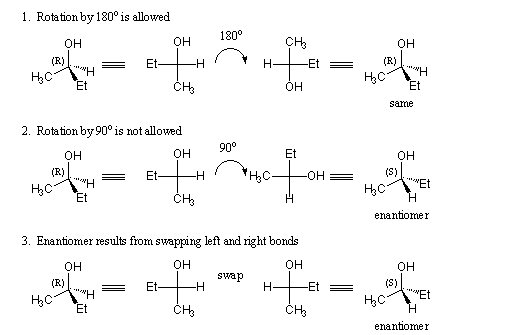
When working with Fischer Projections, keep in mind the following rules:
- Because the "up" and "down" aspects of the bonds don't change, a Fischer projection may be rotated by 180 degrees without changing its meaning.
- A Fischer projection may not be rotated by 90 degrees. Such a rotation typically changes the configuration to the enantiomer.
- To find the enantiomer of a molecule drawn as a Fischer projection, simply exchange the right and left horizontal bonds.
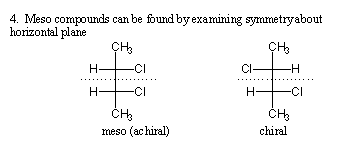
- To determine whether the molecule in Fischer projection is a meso compound, draw a horizontal line through the center of the molecule and determine whether the molecule is symmetric about that line.
To determine the R and S configuration of the chiral carbon atoms in a Fischer projection, we need first recall the concept of the Fischer projection. And that is; the horizontal groups are pointing towards the viewer (wedge), and the groups on the vertical axis are pointing away from the viewer (dash) even though all the bonds are shown in plain lines.
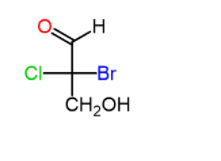
For example, lets determine the configuration of the chiral carbon in the following Fischer projection:
Step 1. Draw the horizontal bonds as wedge lines:
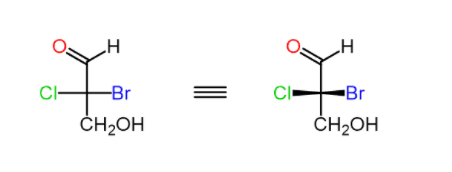
Step 2. Assign the priorities of the four groups:
Determining Priorities on Fischer Projections
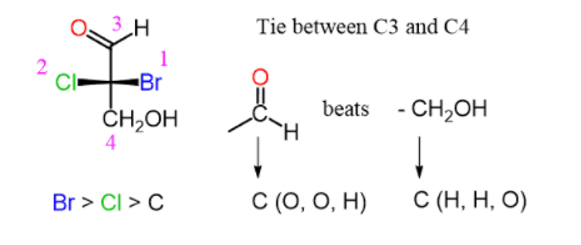
Notice that the aldehyde group has a higher priority than the alcohol because the C=O double is counted as if the carbon is connected to two oxygen atoms.
Step 3. Determine the direction of the arrow; if the lowest priority is pointing away from you (vertical position), then the configuration is as it should be: clockwise (CW)-R, counter clockwise (CCW)-S:
In this case, the CH2OH group is the lowest priority and pointing away from us therefore, the configuration is based on the direction of the arrow.
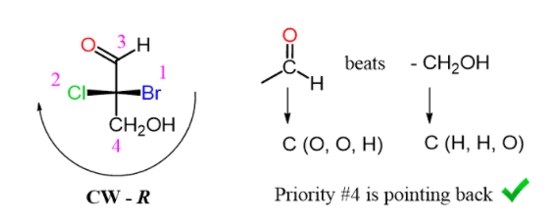
Let’s now consider an example where the lowest priority is on a horizontal position (wedge line):
Step 1. Draw the horizontal bonds as wedge lines:
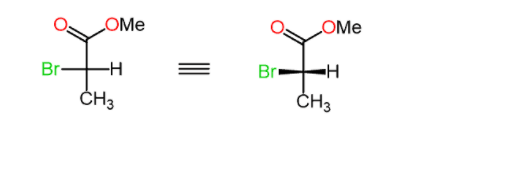
Keep in mind that the lowest priority is pointing towards as.
Step 2. Assign the priorities:
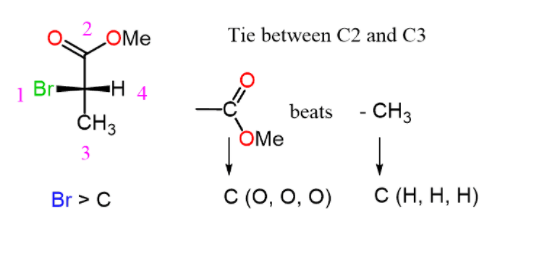
Step 3. Determine the direction not the arrow and change the result (R to S or S to R) because the lowest priority is pointing towards as:
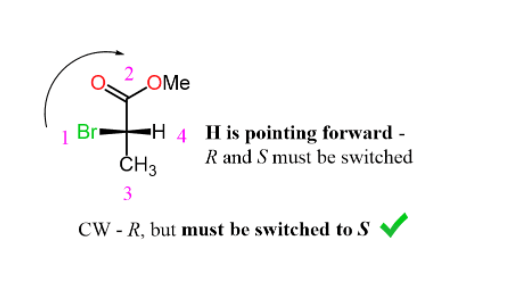
Determining R and S configuration on a Fischer Projection
Step1: Draw the horizontal bonds as wedge lines:
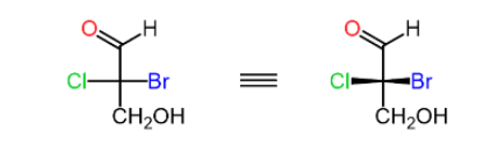
Step 2: Assign the priorities of the four groups
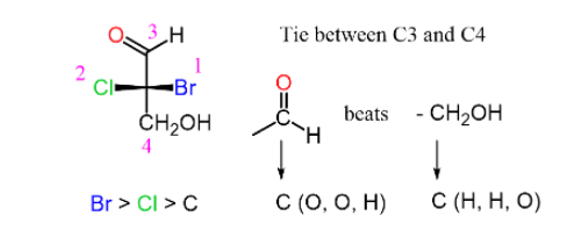
Step 3: Determine the direction of the arrow
If the lowest priority is pointing away from you (vertical position) then the configuration is as it should be: clockwise (CW) -R counter clockwise(CCW) -S
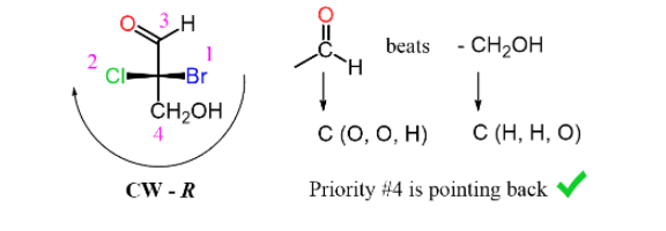
If the lowest priority is pointing towards you (horizontal position) the result must be changed (R to S or S to R):
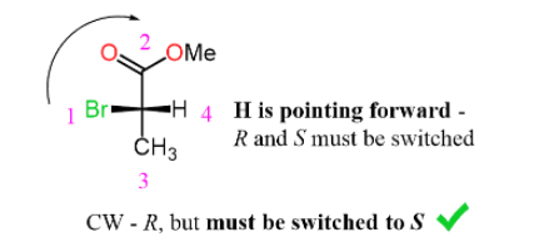
Determining R and S configuration on Fischer Projection
Step 1: Determine the horizontal bonds as wedge lines:
Step 2: Assign the properties of the four groups
SAWHORSE FORMULA
The sawhorse formula indicates the arrangement of all the atoms or groups on two adjacent carbon atoms. The bonds between the two carbon atoms are drawn diagonally and of relatively greater length for the sake of clarity. The lower left-hand carbon is taken as the front carbon or towards the observer and the upper right-hand carbon as the back carbon or away from the observer. e.g., ethane
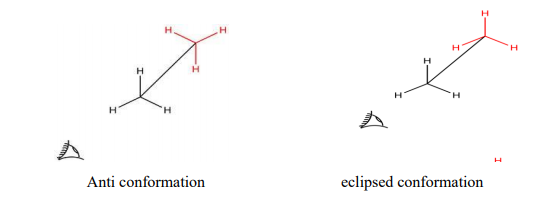
All parallel bonds in sawhorse formula are eclipsed and all anti parallel bonds are opposite or scattered. Gauche representation is that in which bulky groups are nearer to each other at 600 angles.
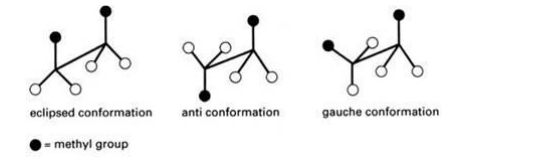
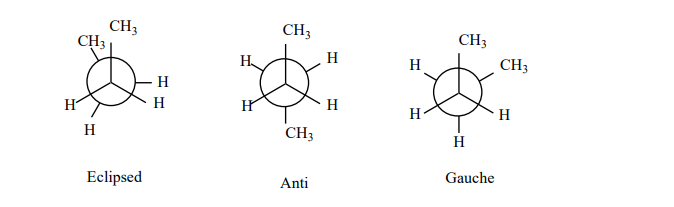
NEWMAN PROJECTION
Newman devised a very simple method of projecting three dimensional formulas on two-dimensional paper which are known as Newman projection.
• In these formulae the molecule is viewed from the front or along the axis of a carbon-carbon bond.
• The carbon nearer to the eye is represented by a point and the carbon atom towards the rear by circle.
• The three atoms or groups on the carbon atoms are shown as being bonded to dot or circle by an angle of 1200 to each other.
• In Newman formula all parallel bonds are eclipsed or all anti parallel or opposite bonds are staggered.
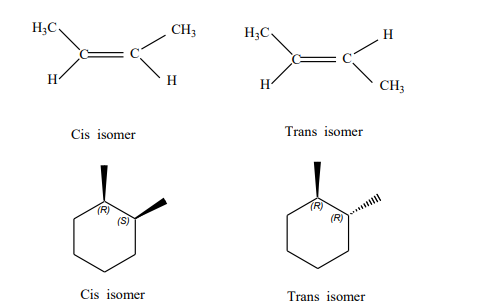
Cis–trans and, syn-anti Isomerism
The Geometrical isomerism is a kind of stereoisomerism, that have the same structure and same molecular formulae but are different in their relative arrangement of atoms. This type of isomerism mainly occurs in heteroleptic complexes and also due to the different possible geometric arrangements for the ligand.
Many diazenes and diphosphates are known to have cis-trans isomers. The Coordination complexes that have square planar or octahedral geometries are also known to display cis-trans isomerism based on the position of the ligands. When adjacent positions are occupied by two identical groups, the isomer is known as cis and when the identical groups are arranged opposite to one another they are called trans.
When geometric isomerism results from a restricted rotation about a carbon-carbon bond. This is called cis-trans isomerism.
The kind of isomerism is seen in a variety of compounds like compounds that contain double bond C=C, C=N, N=N, Cyclic structure compounds, or compounds that contain restricted rotation due to steric hindrance
Syn and anti are identical to Z(usammen) and E(ntgegen), the terms are used frequently to describe the geometry about carbon-nitrogen double bonds. In such cases, the lone pair of electrons is given the lowest priority and the sequence rule applied as usual. For example, in the following N-methyl imines, the imine on the left has the highest priority group attached to the carbon in the double bond (ethyl) on the same side as the highest priority group attached to the nitrogen in the double bond (the methyl has higher priority than the lone pair). Therefore, the molecule is the syn isomer (but would now be called the Z isomer).
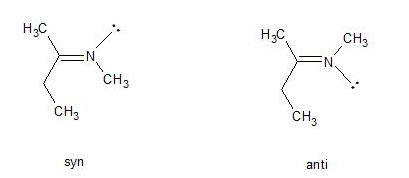
For the same reasons, the molecule present on the right is the anti of E isomer, the key factor here is the lone pair has a lower priority than any other atom.
Among steroids there is another use of the term’s anti and syn that is very prevalent. While the relationships between rings A and B/ B and C are described as trans in tricycles 1 and 2, the relationship between rings A and C in the former is anti while in the latter it is syn.
E/Z Notation
E-Z Notation: The E/Z notation is a method adopted, as the simple denotion of cis/trans in geometric isomerism is not sufficient, as there are more than two different substituents on the double bond, therefore to differentiate the stereochemistry in them this new system of nomenclature is introduced.
According to this method, when groups which have priority are present in the opposite sides of the double bond, that isomer is denoted by E.
Where E = Entgegen ( the German word for 'opposite')
However, if the groups with higher priorities are on the same side of the double bond, that isomer is denoted by Z.
Where Z = Zusammen (the German word for 'together')
The letters E and Z are represented within parentheses and are separated from the rest of the name with a hyphen.
Step by step procedure to determine the E-Z configuration:
The following procedure is to be adopted to denote the geometrical isomers by E & Z descriptors.
- The first step is to establish the group of higher priority present on each side of the double bond.
- If the groups with higher priority are present on the opposite sides of the double bond, the isomer is symbolised by the descriptor, E.
- If the groups are present on the same side of the double bond, the Z descriptor must be used.
The priorities are assigned by following Cahn-Ingold-Prelog sequence rules (CAN rules) described below.
- High priority is given to the atoms with high atomic number and the atoms are ranked according to those attached directly to the olefinic carbon according to their atomic number
- The high priority is given to the isotope that possess high atomic mass when isotopes of same element are present.,
E.g., Deuterium isotope (H2 or D) has more priority than protium (H1 or H).
The C13 isotope has more priority than C12.
If atoms are found to be still identical, the atoms present next along the chain is considered until a “first point of difference” is found. This is however achieved by making a list of the atoms that are linked directly to the first atom, they are arranged in decreasing order of their atomic number. Then the higher priority is given to the list which contain atom with higher atomic number at first point of difference.
E.g., Examine the lists of atoms directly linked to the highlighted carbons in the following compound, (2Z)-2-tert-Butyl-3-methylpent-2-en-1-ol.
The (C, H, H) list has more priority over (H, H, H). Whereas, the (O, H, H) has more priority over (C, C, C). Since the groups with highest priorities are on the same side of the double bond, the descriptor, Z is used to represent the stereochemistry of groups at double bond.
*The multiple bonds are counted as multiples of that same atom i.e., each π bond is treated as if it were another σ bond to that type of atom.
1) The cis and trans 2-butenes can also be represented as Z and E isomers respectively.
Since the double bond is at 2nd carbon of the butene, the number 2 is used before the descriptors i.e., '2Z' or '2E
C.I.P Rules
The method of unambiguously assigning the handedness of molecules was originated by three chemists: R.S. Cahn, C. Ingold, and V. Prelog and, as such, is also often called the Cahn-Ingold-Prelog rules. In addition to the Cahn-Ingold system,
Before applying the R and S nomenclature to a stereocenter, the substituents must be prioritized according to the following rules:
Rule 1
Firstly, the atoms that are attached directly to the stereocentre of the compound has to be examined., the substituent that has a high atomic number will be preferred over the substitute with a low, Hydrogen is the possible lowest possible priority substituent as it has the lowest atomic number. When dealing with isotopes, the atom with the higher atomic mass receives higher priority.
- When a molecule is visualised, the substituent with lowest priority, should always point away from the viewer, (a dashed line indicates this). To understand how this works or looks, imagine that a clock and a pole. Attach the pole to the back of the clock, so that when looking at the face of the clock the pole points away from the viewer in the same way the lowest priority substituent should point away.
- Then, draw an arrow from the highest priority atom to the 2nd highest priority atom to the 3rd highest priority atom. Because the 4th highest priority atom is placed in the back, the arrow should appear like it is going across the face of a clock. If it is going clockwise, then it is an R-enantiomer; If it is going counter clockwise, it is an S-enantiomer.
When looking at a problem with wedges and dashes, if the lowest priority atom is not on the dashed line pointing away, the molecule must be rotated.
Remember that
- Wedges indicate coming towards the viewer.
- Dashes indicate pointing away from the viewer.
Rule 2
If there are two substituents with equal rank, proceed along the two substituent chains until there is a point of difference. First, determine which of the chains has the first connection to an atom with the highest priority (the highest atomic number). That chain has the higher priority.
If the chains are similar, proceed down the chain, until a point of difference.
For example: an ethyl substituent takes priority over a methyl substituent. At the connectivity of the stereocenter, both have a carbon atom, which are equal in rank. Going down the chains, a methyl has only has hydrogen atoms attached to it, whereas the ethyl has another carbon atom. The carbon atom on the ethyl is the first point of difference and has a higher atomic number than hydrogen; therefore, the ethyl takes priority over the methyl.
Rule 3
If a chain is connected to the same kind of atom twice or three times, check to see if the atom it is connected to has a greater atomic number than any of the atoms that the competing chain is connected to.
- If none of the atoms connected to the competing chain(s) at the same point has a greater atomic number: the chain bonded to the same atom multiple times has the greater priority
- If, however, one of the atoms connected to the competing chain has a higher atomic number: that chain has the higher priority.
Optical Activity
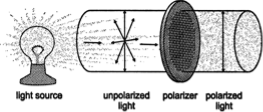
During the nineteenth century a French physicist Jean Biot discovered an important factor in the stereochemistry of molecules. During one of his research works, Biot was studying the nature of plane -polarized light and discovered that solutions of some organic compounds caused polarized light to rotate. Compounds with this property is called optically active, and the amount and direction of rotation can be determined with a polarimeter.
Plane polarized light
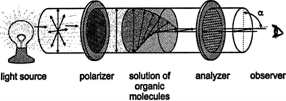
Amount and direction of rotation can be determined with a polarimeter
The white light contains electromagnetic waves that oscillate in an infinite number of planes at right angles to the direction where the light travels. Plane‐polarized light is ordinary white light that has passed through a polarizer. A polarizer is a filter that blocks the light waves in all planes but one. A common example is a pair of Polaroid sunglasses, which prevent glare by polarizing sunlight.
The enantiomers generally have properties like physical properties, like boiling point, melting point, refractive index and density, for chemists, who are working on chiral compounds find it difficult to experiment on enantiomers. However, enantiomers differ in one physical property that is their ability to rotate plane-polarized light.
The light that we see is typically unpolarized, which means that they consist of waves that are oriented in every direction in an even distribution. We can pass unpolarized light through a polarizing filter to obtain plane-polarized light, which consists of light waves oriented in only a single direction.
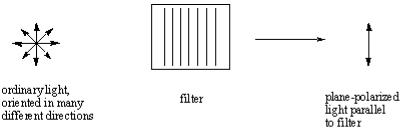
Plane-polarized light
The rotation of plane polarization is seen in solutions of chiral compounds, when light is passed through them, that is the angle of the light plane tilts towards the right and left after it comes out of the sample. However, this property is absent in Achiral compounds. The ability of a solution to rotate plane-polarized light in this fashion is called optical activity, and solutions which have this ability are said to be optically active.

Rotation of plane-polarized light by optically active compounds
The polarimetry technique is used to measure the optical activity through a device called Polarimeter. Using a technique called polarimetry, optical activity is measured by a device called a polarimeter. Monochromatic light (light containing a single colour) is filtered through a polarizer to produce plane-polarized light, and it is passed through the sample. A second filter is placed with its slits parallel to those of the first filter, then the sample is rotated until light is transmitted through the second filter. The number of degrees the sample is rotated is called the optical rotation of the sample. If rotation occurs to the right (clockwise), the optical rotation is given a + sign and the sample is considered dextrorotary. If rotation occurs to the left (counter-clockwise), the optical rotation is assigned a--sign and the sample is laevorotary.
The optical rotation of a given sample varies with its concentration and the light's path length:
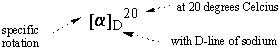
Dependency of the optical rotation on concentration and path length
The proportionality constant [α] is the property of the chiral compound that is characteristic to a particular compound with fixed wavelength and temperature. The constant is called the specific rotation of the compound. Chemists have compiled a large volume of specific rotation data, using as standard conditions
The optical rotation is the angle through which the plane of polarization is rotated when polarized light passes through a layer of liquid.
Optical activity is the ability of a compound to rotate the plane of polarized light. This property arises from an interaction of the electromagnetic radiation of polarized light with the unsymmetric electric fields generated by the electrons in a chiral molecule.
A compound is said to be optically active when the linearly polarized light is being rotated when it is passing through it. The optical rotation is the angle through which the plane of polarization is rotated when polarized light passes through a layer of a liquid. Optical rotation is the effect which is determined by the concentration of chiral molecules and their molecular structure in a substance. Every optical active substance has its own specific rotation.
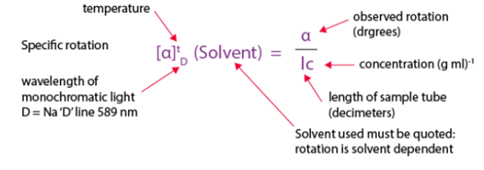
The specific optical rotation of a liquid substance is the angle of rotation measured as specified in the monograph, calculated with reference to a layer 100mm thick and divided by the relative density measured at the temperature at which the rotation is measured.
A measure of the optically activity of a sample is the rotation produced for a 1mm slab for a solid or a 100mm path length for a liquid. This measure is called the specific rotation. Liquids usually rotate the light much less than solids. Solutions of solids will obviously show an effect that depends on the concentration of active material and to a small extent, both on temperature and the solvent.
The Formula of Optical Rotation
Optical activity is the ability of a compound to rotate the plane of polarized light. This property arises from an interaction of the electromagnetic radiation of polarized light with the unsymmetric electric fields generated by the electrons in a chiral molecule.
The rotation observed will clearly depend on the number of molecules exerting their effect that depends upon the concentrations. Observed rotations are thus converted into specific rotations that are a characteristic of the compounds according to the formula below.
Applications of Optical Rotation
- Optical rotation is a function of time and it is used to determine kinetic reactions
- This is also used to plot optical rotatory dispersion curves for a various range of wavelengths this helps in analysing molecular structure
- The optical rotation is measured on a layer of suitable thickness at the wavelength specified in the monograph.
- If the specific rotation of a sample is known its concentration in the solution can be estimated.
- If the concentration of the material in the sample is known then its specific rotation can be determined.
- The technique may be extended to the determination of optical substances in the presence of optically inactive species.
Chirality is defined as “an object which is asymmetric and cannot be superimposed over its mirror image is known as chiral or stereocenter”. This property is known as chirality.
For example- legs and our hands etc.
The object which is symmetric in nature and can be superimposed over its mirror image is known as achiral.
For example- cube, cone etc
Organic compound are known to exhibit the chirality behaviour, this kind of behaviour is due to the spatial arrangement of molecules or three dimensional structure. Dutch scientist, J. Van’t Hoff and a French scientist, C. Le Bel in the year 1874, they exhibited the spatial arrangement of four groups around a central carbon is tetrahedral and if all the substituents attached to that carbon are different; such carbon is called asymmetric carbon or stereo centre. The resulting molecule would lack symmetry and is referred to as asymmetric molecule. The asymmetry of the molecule or chirality is responsible for the optical activity in such organic compounds.
The symmetry of a molecule describes how its different parts relate to one another geometrically. Symmetry plays an important role in many areas of chemistry, with effects on:
- Physical properties: e.g., dipole moment, chirality
- Spectroscopic properties: geometric equivalence of groups or nuclei, transition intensities
- Bonding interactions: the bonds need to overlap of atomic orbitals of correct symmetry.
Enantiomers are a pair of molecules that exist in two forms that are mirror images of one another but cannot be superimposed one upon the other.
Enantiomers are in every other respect chemically identical. A pair of enantiomers is distinguished by the direction in which when dissolved in solution they rotate polarized light, either dextro (d or +) or levo (l or -) rotatory.
Structure of Enantiomers
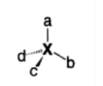
Consider the most common origin of chirality is when a tetrahedrally coordinated atom is bound to four different substituents as shown below.
- Enantiomers were introduced as stereoisomers that are non-superimposable mirror images of one another.
- Any molecule that is not superimposable on its mirror image and so exists as a pair of enantiomers is said to be chiral and to exhibit chirality. Conversely, any molecule that is superimposable on its mirror image is achiral.
- Indeed, whenever a molecule contains a single atom which is tetrahedrally bound to four different substituents, then two enantiomers are possible.
- It is however important that the four substituents are different to one another as if any two of them are identical then the structure would become superimposable on its mirror image and so achiral. The atom connected to four different atoms is best referred to as a stereo genic centre or simply a stereocenter.
- A widely used although somewhat misleading alternative name for a stereocenter is a localized around the central atom, whereas chirality is a property of the molecule as a whole that cannot be localized around one atom or a group of atoms.
- The presence of a stereocenter is not a requirement for a molecule to exhibit chirality; it is simply the most common cause of chirality.
Properties of Enantiomers
- Enantiomers generally have identical physical properties such as melting point, boiling point, infrared absorptions and NMR spectra.
- It is important to realize however, that whilst the melting point etc of one enantiomer will be identical to that of the other enantiomer, the melting point of a mixture of the two enantiomers may be different.
- This is because the intermolecular interactions between opposite enantiomers that are between the R and S enantiomers may be -different to those between like enantiomers that are between two molecules both of R or both of S stereochemistry.
- The one class of physical techniques that can distinguish between the two enantiomers of a compound are chiroptical techniques, the most common of which is optical rotation.
- The chiroptical properties of a molecule are determined not just by the bond lengths and angles but also by the sign and magnitude of the torsional angles, the sign of the torsional angles being the one difference between enantiomers
When an organic molecule possesses, any carbon atom with 4 different substituents, such a molecule will be a chiral centre.
Consider the following molecule:
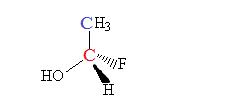
The molecule contains 2 carbon atoms one in red and the other in blue.
3 Hydrogen atoms and 1 carbon atom is attached to the blue carbon atom.
As only substituents (H and C) are attached to this carbon atom, this carbon atom is NOT a chiral centre.
When we consider the red carbon C
1 OH group,1 H atom and one F atom and one methyl group is attached to this carbon atom
There are 4 different substituents on this carbon atom.
This carbon atom is a chiral centre.
Therefore this molecule will be chiral.
Consider the molecule 2-bromobutane shown below with carbon atoms numbered 1, 2, 3 and 4:
| H |
| Br |
| H |
| H |
|
H- | C1 | - | C2 | - | C3 | - | C4 | -H |
| | |
| | |
| | |
| | |
|
It is clear that carbons 1 and 4 cannot be chiral centres because they have 3 hydrogen atoms bonded to them so they do not have 4 different substituents.
Similarly carbon 3 cannot be a chiral centre because it has 2 hydrogen atoms bonded to it so it does not have 4 different substituents and cannot therefore be a chiral centre.
But what about carbon 2? Is it a chiral centre?
Let's redraw the structure to make it clearer...
|
| Br |
|
|
H3C | - | C2 | - | CH2CH3 |
|
| | |
|
|
Now we can clearly see that carbon 2 has 4 different substituents:
- a methyl group (CH3)
- a bromine atom (Br)
- a hydrogen atom (H)
- An ethyl group (CH2CH3)
Distereoisomers are defined as compounds which have the same molecular formula and sequence of bonded elements but which are nonsuperimposable, non-mirror images.
Enantiomers and diastereomers commonly called stereoisomers fall under the broader concept of isomerism which always involves the comparison of at least two species.
Stereoisomers are molecules of identical constitution but nevertheless different. Differences between diastereomers can be expressed in scalar terms, that is by differences in the distances of certain characteristic pairs of atoms.
Stereoisomers that are not related as an object and its mirror image are called diastereomers; Diastereomers are stereoisomers that are not mirror images.
Let us consider the formula of 2,3-dichloropentane which contains two chiral centres. Now compare the two forms of 2,3-dichloropentane given below.

It becomes immediately clear that the two formulae have identical configurations about one chiral carbon C2 and mirror image configurations about the other chiral carbon (C3). The net result is that the two forms are neither identical nor mirror images of each other. Such stereoisomers of a substance that are not mirror images of each other are known as diastereomers.
Characteristics of Diastereomers
- Diastereomers have different physical properties such as melting points, boiling points, densities, solubilities, refractive indices, dielectric constants and specific rotations. Enantiomers have similar physical properties except the opposite sign of specific rotation.
- Diastereomers other than geometrical isomers may or may not be optically active.
- Diastereomers show similar, but not identical chemical properties. The rates of reactions of the two diastereomers with a given reagent provided the reagent is not rapidly active.
- On account of differences in their physical properties, diastereomers can be separated from one another through techniques like fractional crystallization, fractional distillation, chromatography etc. The difference from enantiomers which can’t be separated by these techniques
A meso compound is one that has one or more stereocentres, and it’s an achiral compound having chiral centres. They are the superimposed on their mirror images and are optically inactive in spite of their stereocentres.
Introduction
Generally, the meso compound must have two or more identical substituted stereo centres., there is also an internal symmetry present that divides the compound into half, resulting in the reflection of the two halves by each other by the internal mirror. The stereochemistry of stereocenters should “cancel out”. Meaning, when the internal plane splits the compound into two symmetrical sides, the stereochemistry of both left and right side should be opposite to each other, and therefore, result in optically inactive. Cyclic compounds may also be meso.
Identification
If A is a meso compound, it should have two or more stereocenters, an internal plane, and the stereochemistry should be R and S.
- Look for an internal plane, or internal mirror, that lies in between the compound.
- The stereochemistry (e.g., R or S) is very crucial in determining whether it is a meso compound or not. As mentioned above, a meso compound is optically inactive, so their stereochemistry should cancel out. For instance, R cancels S out in a meso compound with two stereocenters.
Trans-1,2-dichloro-1,2-ethanediol
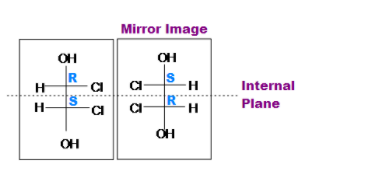
(meso)-2,3-dibromobutane
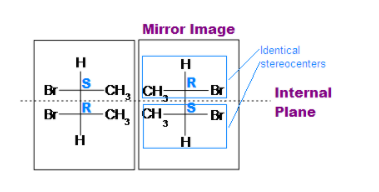
There is organic compound that have similar chemicals formulas but different molecular structures. They are called enantiomers. When enantiomers are present in equal quantities in a mixture, it is called a racemic mixture. The description of this mixture shows how structural and molecular structure can vary due to the presence of functional groups in a different orientation. Let us discuss the topic elaborately and understand the meaning of the terms with examples
The enantiomers are compounds have the same chemical formula but different molecular orientations making them a particular set of isomers, they show adifferent set of physical properties but similar chemical properties in most cases
The only way to differentiate between two enantiomers is to pass a polarized light beam and check the changes in its plane.
The functional groups in a molecule of a compound can either be in the right or the left position in the same location. Their orientation might vary in the same carbon position in a molecular structure. The chemical properties are alomost the same for both compounds. The mixture o these two compounds is called racemate or racemic mixture.
As per the racemate chemistry, the proportion of two enentiomers in the mixture is equal. The name comes from the first mixture to be recognized by louis Pasteur, he identified the presence of two types of isomers in racemic acid, it is also called tartaric acid. The term racemic comes from the latin word “racemus” which means a bunch of grapes. This right -oriented molecule is known as tartaric acid.
When Louis pasteur identified the presence of both the acids in the same mixture in equal propotion he named it a racemic mixture, this is where the name is derived from, both the compounds are similar in chemical structure. The only difference is the presence of one of two functional positions in two different orientations.
Nomenclature of Racemate compounds
In racemate chmistry , when a mixture is not showing any effect or change ina polarized light passing through it, it means that the quantities of both the compounds are equalwhen these compounds are separated are separated and then a polarization light is passed , a particular change is noticed. The light either rotated anticlockwise or clocwise. When there is an anticlockwise rotation of the plane-polarized light.it is called dextrorotation. Similarly when there ic clockwise rotation of plane polarised light is called levorotation. Hence for the compounds showing any of the two rotatory effects , the chemical name of the compounds comes with a specific prefix.
According to the racemic meaning and nomenclature, a dextrorotary compound with a ‘+’ ‘d’ or’D’ predfix all are the same meaning, similarly a levorotatory compound comes with the prefix ‘-‘ ‘l’ ‘L’. For e.g Dextro-fructose can be presented as D-fructose , +fructose or d-froctose, similarly an enantiomer with levorotation can be represented as L-fructose, -fructose, or l-fructose.
Hence these optically active eneatiomers can be distinguished and named using their optical activities. The prime feature of two such compounds of similar chemical formula but different molecular struvcute is that the degree of rotation occuring in the plane-polarised light will have the same angulas value but in opposite directions, this is why the + and – signs are used to signify them.
The chiral compounds are synthesised by starting materials that are achiral in nature and the reagents are usually racemic, (i.e., a 50:50 mixture of enantiomers). Separation of racemates into their component enantiomers is a process called resolution. Enantiomers have similar properties like solubility, melting point and resolution are very difficult, where Diastereomers on the other hand have different physical properties, and this is the concept used to achieve resolution of racemates. The reaction of a racemate with a pure chiral enantiomer reagent results in a mixture of diastereomers, that can be separated., thus reversing the first reaction leads to the separated enantiomers plus the recovered reagent.
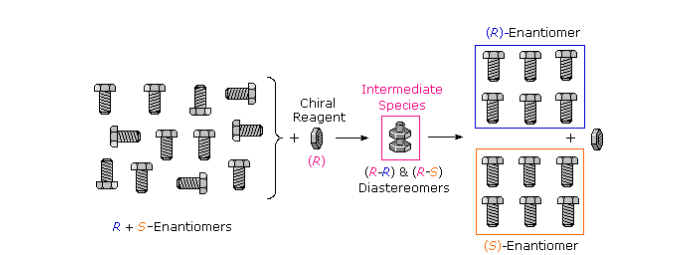
Many kinds of chemical and physical reactions, including salt formation, may be used to achieve the diastereomeric intermediates needed for separation. Figure illustrates this general principle by showing how a nut having a right-handed thread (R) could serve as a “reagent” to discriminate and separate a mixture of right- and left-handed bolts of identical size and weight. The fully threaded -intermediate, can occur only if the two right-handed partners, so separation is fairly simple. The resolving moiety, i.e., the nut, is then removed, leaving the bolts separated into their right and left-handed forms. Chemical reactions of enantiomers are normally not so dramatically different, but a practical distinction is nevertheless possible.
Because the physical properties of enantiomers are identical, they seldom can be separated by simple physical methods, such as fractional crystallization or distillation. It is only under the influence of another chiral substance that enantiomers behave differently, and almost all methods of resolution of enantiomers are based upon this fact.
The phenomenon of inversion of configuration during a chemical reaction is known as Walden Inversion. Generally, Walden inversion is referred to as optical inversion. The inversion of configuration may or may not lead to the change in direction of rotation.
Walden’s inversion is the reversal of a chiral center in a molecule in a chemical reaction. Since the molecule can form two enantiomers around the chiral center, the Walden inversion transforms the structure of the molecule from one enantiomeric form to another.
Walden Inversion Reaction
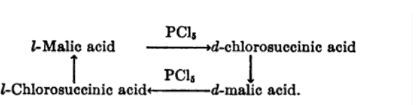
In 1896, Walden reported an inversion of optical rotation in the conversion of malic to chlorosuccinic acid by reaction with phosphorus pentachloride.
Walden inversion has been extensively studied with numerous reactants and optically active substances. Perhaps the most satisfactory explanation for the change in configuration was suggested by Werner in 1911 and is commonly known as the opposite face mechanism for the Walden inversion.
For example,
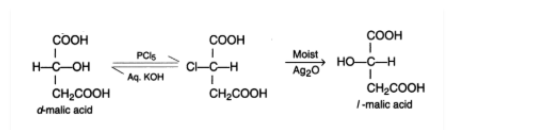
A Walden inversion occurs at a tetrahedral carbon atom during an SN2 reaction when the entry of the reagent and the departure of the leaving group are synchronous. The result is an inversion of configuration at the centre under attack.
Walden Inversion Mechanism
The stereochemical course of an SN2 reaction’s inversion of configuration is explained below.
Nearly 100 years ago, Paul Walden demonstrated that (+) malic acid could be converted to either (+) or (-) chlorosuccinic acid (2-chlorobutanedioic acid) with different reagents. Although the absolute configuration of each substance was not known at the time, it was clear that one of these processes occurred by inversion of configuration at the stereocenter and the other by retention.
A series of investigations definitely showed that the stereochemistry of a chiral substance is normally inverted during the course of an SN2 reaction.
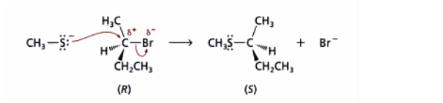
To commend Walden for his work on the stereochemical course of substitution reactions, we sometimes refer to the stereochemistry of an SN2 reaction as Walden inversion.
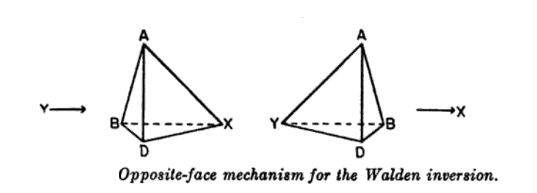
The presence or absence of an asymmetric or a chiral carbon atom in a molecule is not only a criterion of dis-symmetry or chirality and hence, enantiomers but it is certain that most of the molecules which have chiral carbon atoms are optically active.
Walden inversion was to develop a scheme for deciding by which mechanism a particular reaction had proceeded or would proceed. Ingold and co-workers showed how to distinguish whether a substitution occurred by the synchronous or the sequential route by kinetic criteria. They then went on to explore the structural features and reaction conditions that promoted one or other of these mechanistic routes.
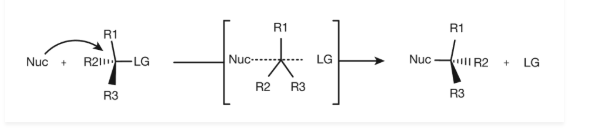
Basically, which mechanism occurs is decided by which transition state is lower in energy. This can be analysed in structural terms, concerning things such as the energetic cost of breaking the initial bond, the steric environment of the transition states and the potential stabilizing effects of the proposed solvent etc. Typically, in synthesis one wants to employ the stereospecific SN2 mechanism because it gives a single predictable product.
Walden Inversion Explanation
Stereochemistry refers to the arrangement of atoms in space, and most particularly to the “handedness” of chemical compounds. When a carbon atom is bound to four distinct groups, those groups are arranged about the carbon as if they were at the vertices of a tetrahedron with the carbon at its center. As a result, there are two distinct such arrangements possible – these are non-superimposable mirror images of one another called enantiomers. Enantiomers have very similar chemical structures and so their chemical and physical behaviour is also very similar.
However, there are some chemical differences between them, and they have different optical activities, they rotate plane polarized light in opposite directions. Walden has shown that it was possible by a series of reactions to convert an optically active substance into its enantiomer. Chemists at the time had assumed that substitution reactions of the sort Walden employed would proceed in a way that either kept the spatial arrangement the same, or perhaps randomized the spatial arrangement.
Walden showed that at some point during his series of reactions, the groups around carbon had to systematically shift from one spatial arrangement to another. In the forty years after the discovery of Walden inversion, many additional inversions were discovered, however, not all substitution reactions on optically active starting products led to inversion, some resulted in the retention of configuration and others led to a mix of enantiomers products.
The relative configuration of molecule is exhibited in the D-L system, and is compared to the glyceraldehyde as the standard compound. Compounds with the same relative configuration as (+)-glyceraldehyde are assigned the D prefix, and those with the relative configuration of (-)-glyceraldehyde are given the L prefix.
This system does not emphasise on the optical activity of the molecule and also does not have a direct connection to the to (+)/ (-) notation. The system shows only a relation between the stereochemistry of the compound with that of glyceraldehyde. We may have compound with same relative configuration as (+)-glyceraldehyde (thus, it's given the D prefix), yet it rotates the polarized light counter clockwise (-), such as D- (-)-ribose.
The D-L system (also called Fischer–Rosanoff convention) is mainly useful in naming naming α-amino acids and sugars. The system compares the relative configurations of the molecules to the enantiomers of glyceraldehyde.
In 1966 Rosanoff selected the enantiomeric glyceraldehyde’s as the standard reference; the D series represents the sugar derived chain lengthening also known as (+)-glyceraldehyde (or named D-glyceraldehyde). In other words, we used a D to designate the sugars that degrade to (+)-glyceraldehyde and an L for those that degrade to (-)-glyceraldehyde.
When we assign D and L configurations of the sugars, the oh groups of the bottom asymmetric carbon is considered., if the group is located in the right it is designated as D and when present in the left side it is designated as L as they have the same relative configurations with the standard glyceraldehyde for the bottom of the asymmetric carbon.
S/R configuration
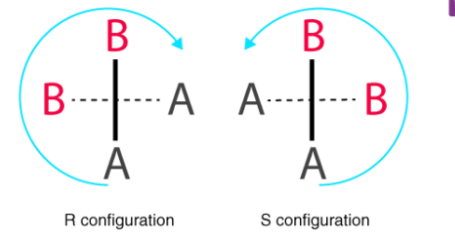
The key difference between R and S configuration is that the R configuration is the spatial arrangement of R isomer, which has its relative direction of priority order in a clockwise direction whereas S configuration is the spatial arrangement of S isomer that has its relative direction of priority order in an anticlockwise direction. Here, the relative direction of the priority order is the descending order of priorities of substituents.
The isomers R and S have chiral centres and are organic molecules, the chiral centres include the carbon atom that has four different substituents attached to it.
The optical rotation concept helps to claim the benefits of the R/s System. The absolute configuration of an enantiomer cannot be established at the same temperature for two enantiomers of a chiral molecule. This is because the sign of optical rotation for a particular enantiomer may change when the temperature changes.
Stereocenters are labelled R or S
The "right hand" and "left hand" nomenclature is used to name the enantiomers of a chiral compound. The stereocenters are labelled as R or S.
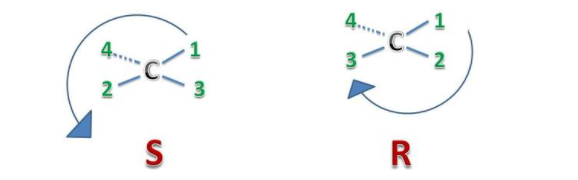
Consider the first picture: a curved arrow is drawn from the highest priority (1) substituent to the lowest priority (4) substituent. If the arrow points in a counter clockwise direction (left when leaving the 12 o' clock position), the configuration at stereocenter is considered S ("Sinister" → Latin= "left"). If, however, the arrow points clockwise, (Right when leaving the 12 o' clock position) then the stereocenter is labelled R ("Rectus" → Latin= "right"). The R or S is then added as a prefix, in parenthesis, to the name of the enantiomer of interest.
Key takeaway
The study of stereochemistry focuses on stereoisomers and spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes method for determining and describing these relationships; the effect on the physical or biological properties, CIS-trans (geometric) isomerism exists when there is restricted rotation in a molecule and there are two nonidentical groups on each doubly bonded carbon atom.
References:
1. Graham Solomons T. W., Fryhle, Craig B., Snyder Scott A, Organic Chemistry, Wiley Student Ed, 11th Edition (2013)
2. Jonathan Clayden, Nick Greeves, Stuart Warren, Organic Chemistry, 2nd Edition, Oxford Publisher, 2014.
3. Dhawan, S.N., Pradeep’s Organic Chemistry, (Vol. I and II), Pradeep Publications