Unit - 4
Bioinorganic Chemistry
4.1.1 Introduction
There are several naturally occurring metal complexes that are involved in life processes in some way 1. Calcium is well-known for its role in the development of strong bones and teeth. Calcium, on the other hand, tends to relieve muscle cramps and triggers a variety of reactions in the human body. Magnesium is necessary for our cells to function properly. Magnesium is, in reality, the most abundant mineral within each cello, second only to potassium. Ca2+ and Mg2+, as well as Zn2+, Cu2+, Fe2+, and Mn2+, play a role in biological processes in the nucleus and are present in measurable quantities (10-2 to 10-4 mol) in the cells2. Mg2+ or Mn2+ concentration affects the active configuration of RNA. Enzymes in both plants and animals depend on the energy provided by magnesium to complete their tasks. Magnesium is believed to provide energy by stimulating the development of adenosine triphosphate (ATP), which provides energy to our body's billions of cells. Magnesium acts as a catalyst or activator in this reaction, as well as a variety of other physical processes.
Enzymatic behaviour, for example, in DNA-polymerase, as well as the effects of divalent ions like Mg2+, Mn2+, and Zn2+ on the rate of polymer synthesis, have all been studied.
Finally, we will discuss the progress of models for metal ion sites found in metal complexes of single metal ions with organic ligands that have oxygen, nitrogen, sulphur, or phosphorous donor atoms as donor atoms. Molecular models were first created to analyse the conformational properties of organic compounds. However, due to the presence of the metal ion, which introduces a number of geometries that the metals present in their compounds, and the lack of criteria for interactions with the metal ion, coordination compounds are less studied. Most molecular mechanics programmes handle metal-ligand interactions by either defining a covalent bond between the metal ion and the ligand atom (the "bonded approach") or using electrostatic and Van der Waals forces (the "non-bonded approach")4. This analysis will not examine the methods; rather, it will describe the essence of the issues and the methods that have been devised to address them.
4.1.2 Biological
Outcomes Metals have been around since the dawn of time. In 3000 BC, the Egyptians used copper to sterilise water, in 2500 BC, the Chinese used gold in medicine, and Hippocrates used a variety of metals to treat diseases, including Cu, Fe, Zn, Na, and K.
Metals make up the vast majority of the Periodic Table's elements (about 80 percent), and the majority of them are involved in biological processes or have been used as narcotics. Figure I depict the coordination chemistry of alkali, alkaline-earth, and toxic cations, as well as the aspects of their complexation for which stability constants and selectivity’s have been calculated using macrocyclic cryptates.
For cryptate 3, 85Sr2+, 224Ra2+, and 140Ba2+, applications of the above cryptates for the removal of radioactive cations have been demonstrated. Sr2+/Ca2+ = 4.103, Ra2+/Ca2+ = l.6.102, Ba2+/Ca2+ = l.3.105, and Sr2+/K+ = 4.102, Ra2+/K+= 16, Ba2+/K+= l.3.104. The radionuc1ide is favoured by all. The tests were carried out in vivo (on rats), and the cryptates significantly increased the excretion of radioactive cations in faeces and urine.
Several cryptates were used as heavy metal detoxification agents, with good binding to toxic heavy metals (Cd2+, H2+, Pb2+) and poor binding to biologically important cations (Na+, K+, Mg2+, Ca2+, Zn +). The cryptates 1-3,6-8 were found to be the best for Ca2+ (selectivities Hg2+/Ca2+ from 1013 to 1025, Hg2+/Zn2+ from 1011 to 1019), while the cryptates 2,3,6-10 were found to be the best for Pb2+ (selectivities Pb2+/Ca2+ from 106 to 1014, Pb2+/Zn2+ from 105 to 10 These cryptates 7 may also be used to extract and transport cations, and they have a wide range of applications in analytical chemistry.
Metal ions are believed to be involved in a variety of biological reactions. Metals are still known as essential elements, despite the fact that their functions in living organisms are unclear. Bioinorganic chemistry, or the analysis of metal functions in biological systems using inorganic chemistry knowledge and methods, has advanced dramatically in recent years.
The following is a list of common metal-containing bioactive substances.
The basis of chemical reactions of metalloenzymes are
In many cases, reaction environments are controlled by biopolymers such as proteins, and selective reactions are performed.
1. Coordinative activation (coordination form, electronic donation, steric effect), 2. Redox (metal oxidation state),
3. Information communication, and, in many cases, reaction environments are regulated by biopolymers such as proteins, and selective reactions are performed.
Other than metalloenzymes, metals can behave in a variety of ways.
1. Mg: MgATP
Pumping of Na/K ions,
3. Ca: Metals play important roles in hormone transfer, muscle contraction, nerve transmission, and blood coagulation, to name a few.
(a) Oxidation
Oxidation reactions in living systems are critical to life, and several studies have been conducted on them. The mechanisms of oxygen gas transportation by haemoglobin and mono-oxygen oxidation by iron porphyrin compounds known as P-450 have been extensively researched. The transportation of oxygen gas, which has been researched for many years, is listed below. The iron porphyrins haemoglobin and myoglobin, as well as the copper compound hemocyanin, are involved in the transfer of oxygen from the environment to living organisms' cells. The reversible bonding and dissociation of dioxygen to iron or copper ions is the foundation of this feature. Metals must be in oxidation states and coordination conditions that allow for the reversible coordination of dioxygen to perform these functions. Human and other animal red blood cells contain the iron porphyrin compound haemoglobin.
Haemoglobin has a heme iron structure with four iron porphyrin units and a globin protein. In the hem iron unit, dioxygen is coordinated to ferrous ions and transported in the blood. With four nitrogen atoms of porphyrin and a nitrogen atom of the polypeptide histidine, the Fe (II) ion is penta-coordinated, and when a dioxygen coordinates to it, it becomes hexa-coordinated. As dioxygen is coordinated, the spin state of Fe (II) varies from high to low. Since it is too big to fit in the available space, the high spin Fe (II) is above the plane of porphyrin. The size of the iron ion decreases as the Fe (II) ion becomes low spin after dioxygen coordination, and it only fits into the hole of the porphyrin molecule.
Since it affects the entire protein via the histidine coordinate bond and regulates the basic bond of a dioxygen molecule, this molecular-level movement has piqued interest as an allosteric impact. A macromolecular protein prevents oxidation of the Fe (II) ion of a hem atom, but if the hem iron is removed, the Fe (II) ion is oxidised to Fe (III), and two porphyrin rings are bridged by a peroxide -O22-, which eventually switches to a bridging -O2-structure.
The hem loses its ability to coordinate with the dioxygen molecule when it is in this state. Based on this finding, a synthetic porphyrin was created that can reversibly coordinate to a dioxygen while suppressing dimerization of the iron porphyrin. It was given the name picket fence porphyrin after its three-dimensional shape.
(b) Nitrogen fixation
All life depends on the reaction that transforms nitrogen in the air into ammonia. Nitrogen fixation, or the process of converting atmospheric nitrogen to ammonia, is carried out by Rhizobium in legume roots and bacteria in algae in an anaerobic environment. Before the invention of the Harber-Bosch process, all animals and plants, including humans, relied on biological nitrogen fixation as a source of nitrogen for protein and other nitrogen-containing compounds.
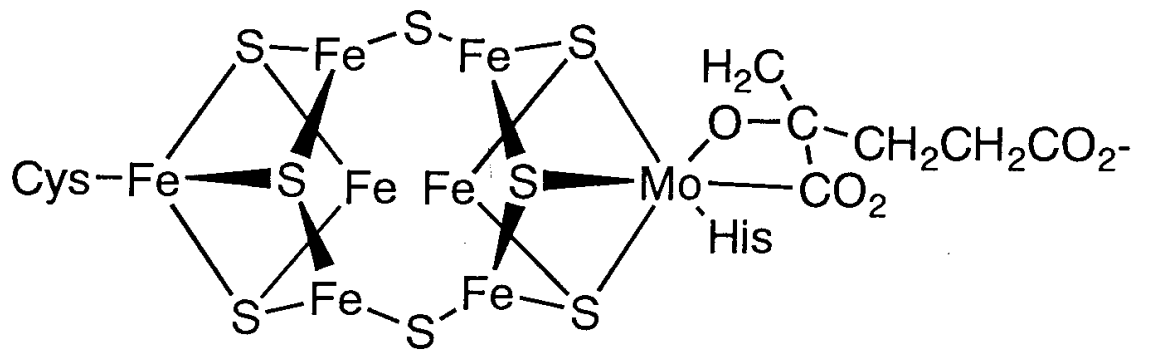
This reaction is catalysed by the enzyme nitrogenase. Nitrogenase is made up of iron-sulfur and iron-molybdenum sulphur proteins, and it uses MgATP as an energy source to reduce dinitrogen through coordination and mutual proton and electron transfers. Because of the significance of this reaction, efforts to decipher nitrogenase's structure and create artificial nitrogen fixation catalysts have been ongoing for several years. Single crystal X-ray research recently revealed the structure of an active centre in nitrogenase called the iron-molybdenum cofactor (Figure 8.2.28.2.2). Fe3MoS4 and Fe4S4 clusters are related through S in this structure, according to this study.
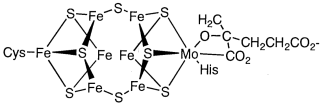
Figure: The Fe-Mo cofactor structure in nitrogenase.
Dinitrogen is thought to be triggered by coordination between the two clusters. The P cluster part, on the other hand, is made up of two Fe4S4 clusters. Both parts' functions and reaction mechanisms are still unknown.
(c) Photosynthesis
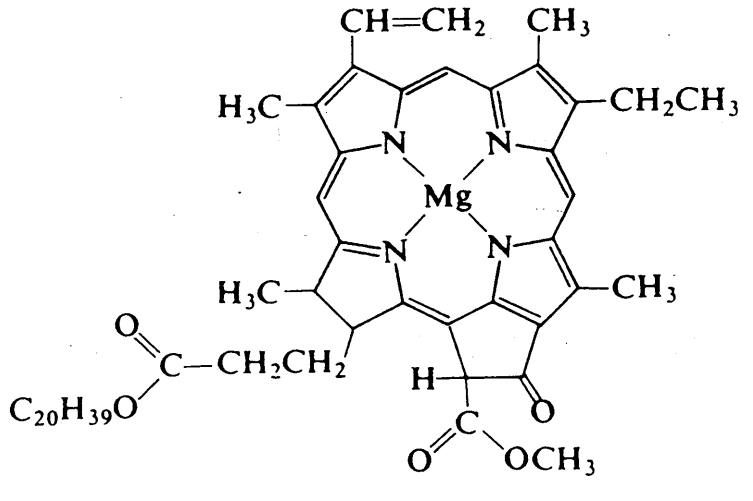
The reaction of carbon dioxide and water to produce glucose and dioxygen is a clever photochemical reaction in which chlorophyll (Figure 8.2.38.2.3), a magnesium porphyrin and manganese cluster complex, plays a key role. Photosystem I (PSI) and photosystem II (PSII) are photosystems that use light energy to eliminate carbon dioxide and oxidise water in chloroplasts.
PSI is made up mostly of chlorophyll. Chlorophyll is a magnesium-based porphyrin complex that gives leaves their green colour. It receives light energy and transfers it to redox reaction systems, which is an essential feature. The energy of the excited state is passed to an acceptor within 10 ps, and the resulting energy reduces an iron-sulfur complex before being used for carbon dioxide reduction in subsequent dark reactions. Since photochemical excitation is the most significant first step in charge separation, studies on photoinduced electron transfer have been conducted extensively using various types of porphyrin compounds as models for chlorophylls. PSI transforms ADP to ATP by obtaining oxidising energy by electron transfer.
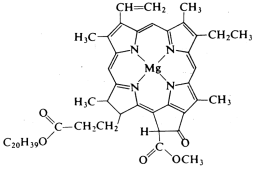
Figure: - Chlorophyll a.
The oxidised form of PSII, on the other hand, oxidises water and produces oxygen through a series of redox reactions involving manganese oxo cluster complexes. At least two manganese species are involved in this reaction because four electrons transfer in the reduction of Mn (IV) to Mn (II). The electron transfer is most likely mediated by a cluster complex containing two Mn (II) and two Mn (IV) species through four step reactions. However, the specifics of this reaction are still unknown due to the difficulty in isolating and analysing this cluster. Various manganese complexes are currently being used as model systems to study the electron transfer point.
Photosynthesis is a fascinating research topic in bioinorganic chemistry because it involves a cycle of subtle electron transfer and redox reactions involving a few metal ions, a porphyrin, sulfide, and oxide clusters that generate oxygen gas by photolysis of water and produce carbohydrates from carbon dioxide via reductive dark reactions. The reaction core of a photosynthetic bacteria was recently crystallised, and J. Deisenhofer and colleagues were awarded the Nobel Prize for their structural research (1988).
Key takeaway:
Introduction
Many scientific disciplines focus on the chemical elements found in organisms and their functions. Such investigations are supported by chemistry and biology. With the passage of time, more advanced disciplines have emerged.
developed with emphasis on either organic or inorganic chemistry.
Investigations of the positions of chemical elements in species combine chemistry and biology.
When organic chemistry is concerned with life and inorganic chemistry is only associated with the inanimate environment, the names of the sub-disciplines may be misleading. Life is made up of both organic and inorganic compounds, and as I'll show later in this article, much more chemical elements are essential for life than those typically studied in organic chemistry. Many metal ions are among them, and they have played an important role in the evolution of life—life would not be possible without them. Metallomics is an applied bio metal science that seeks to cover all facets of how metals work in biological systems.
There are three distinct trends that contributed to our current understanding, all of which are linked to a change in the types of questions answered and are still ongoing.
First, researchers attempted to decide the elements are found in living organisms and which are needed for survival. Except for sulphur, which is just around 100 g in a 70 kg person, the bulk elements hydrogen (H), carbon (C), nitrogen (N), oxygen (O), and sulphur (S) are all over 1 kg in a 70 kg human. Macro minerals were discovered, such as the metal cations sodium (Na), potassium (K), magnesium (Mg), and calcium (Ca), as well as the non-metals phosphorous (P) and chlorine (Cl), all of which are in the gramme category, with the exception of calcium, which is around 1.7 kg. With advancements in analytical chemistry and instrumentation, trace element analysis matured and provided evidence for additional important elements, including the metal ions iron (Fe), zinc (Zn), manganese (Mn), copper (Cu), cobalt (Co) in the form of vitamin B12, molybdenum (Mo), and the non-metals selenium (Se) and iodine (Io) (I). Except for iron and zinc, which are 2–5 g and therefore not trace elements, they are all in the mg range. Other elements are present at even lower levels, but others, such as bromine (Br), rubidium (Rb), aluminium (Al), nickel (Ni), titanium (Ti), and barium (Ba), are present above the levels of basic elements such as Mn and Mo. (Ba).
Second, we saw metalloproteins being isolated and the coordination conditions in which metal ions work being characterised. According to bioinformatics, about half of all enzymes depend on a metal ion for catalysis, demonstrating the importance of metal ions in biochemistry. Around 3000 human proteins have the structural features of zinc metalloproteins, implying that zinc is present in about every tenth protein encoded in the human genome. Metal interactions with biological macromolecules other than proteins in vivo are poorly understood. Magnesium is an exception, since it plays a part in the structure and function of RNA.
Third, membrane metal transporters, metalloregulators, and metallochaperones were identified as proteins with particular roles in metal metabolism. These findings shed light on the intricate biological processes that govern metal control and selectivity. It was discovered that each metal ion has a specific concentration range that is determined by the affinities of metal ions for their ligands. As a result, the binding power (affinity) increases in the sequence Mg, Ca, Mn, Fe, Co, Ni, Zn, Cu, and the free metal ion concentrations (metal ions not bound to proteins) decreases. This sequence is similar to the Irving-Williams series for divalent metal ions in theory. Despite the fact that Mg complexes are normally stronger than Ca complexes, Ca is stabilised in biology by using more ligands and hence coordination numbers greater than six. Since Ca2+ has been adopted as a cellular signalling ion regulating many processes, this stabilisation is required. In the periodic scheme of the elements (PSE), Zn comes after Cu, but Cu complexes are more stable. Cu is the only metal ion in this sequence that is predominantly monovalent (Cu(I)) in the cell. The fact that these ranges of free metal ion concentrations span about 15 orders of magnitude, from millimolar (Mg) to attomolar (At), is a remarkable feature (Cu). Metal ions can thus be used in a wide variety of biological processes, showing the tremendous contribution that metal ions provide to biological function. Human total cellular metal ion concentrations, on the other hand, follow a different pattern: Ca, Fe, Zn, Cu, Mn, Co, Mg, Ca, Fe. As a result, biology must account for the relatively large variations in total metal concentrations in order to preserve the pattern expressed by the Irving-Williams sequence for characteristic free metal ion concentrations. It also has to deal with signalling metal ion fluctuations and oscillations. Zn2+ is a cellular signalling ion that works in tandem with Ca2+. Although Ca2+ appears to be primarily an activator of several processes, Zn2+ appears to be primarily an inhibitory ion that targets various proteins due to its preference for different ligands and coordination environments.
The Essential Elements
Given our vast understanding of genes and proteins, it's amazing that our understanding of the role of chemical elements in life is still minimal and open-ended. Bromine, which plays a role in collagen metabolism, was only recently added to the list of essential elements. As a result, we shouldn't presume that we know everything there is to know about the elements that are important for animals and humans, since certain chemical elements' biochemical functions are still unknown. As startling as this assertion may be, the lack of expertise opens up a plethora of possibilities for biochemistry and health improvement discoveries.
We now know that humans require 11 metals and 10 non-metals, totalling 21 elements. Chromium, whose position as an integral element is debatable, is included in the total (see below). Other modes of life outside the kingdom animalia include additional elements. As a result, the nature and consequences of critical elements specified in a "biological periodic system of the elements (PSE)" are often unclear. Conjectures emerge primarily as a result of two factors:
A reference to the biological species should be included in the term "essential." All of the elements listed as important in all organisms are normally included in biological PSEs. Some components, on the other hand, are required by only a few species. I suggest the following classification: I elements that are common to all species; (ii) elements that are used by a large number of species (V, Ni); and (iii) elements that are used by a small number of organisms in unique ecological niches (W, Cd, Lanthanides). In algae and fungi, vanadium is used in nitrogenases and halo peroxidases. Just nine enzymes in bacteria and plants have been discovered to contain nickel. Some thermophilic bacteria use tungsten instead of molybdenum in their enzymes. Ni, V, and W have not been shown to be important for animals or humans. In both animals and humans, orthologous gene products requiring these elements have not been discovered. In the marine diatom Thalassiosira weissflogii, carbonic anhydrase, which is normally a zinc enzyme, is a cadmium enzyme. Cadmium is listed as a Group B1 potential human carcinogen by the US Environmental Protection Agency (EPA). Lanthanides (rare earth elements, REE) were discovered as cofactors of methanol dehydrogenase in methanotrophic bacteria instead of calcium. While the broader use of metal ions in life is fascinating from an evolutionary perspective and has undoubtedly uncovered fascinating chemistries, the use of basic elements such as Cd and REE is of minimal importance for animal and human nutrition.
A meaning for the term "essential" should be included. It's important to differentiate between elements that are necessary for survival and those that only have minor functions and health benefits. Fluorine, for example, prevents dental caries and can be helpful to bone health, but it is harmful in other ways. “An element is necessary when a deficient intake repeatedly results in an impairment of a feature from optimal to suboptimal, and when supplementation with physiological amounts of this element, but not others, prevents or cures this impairment,” according to the new definition. Several of them, such as B, Cr, Ni, and Si, are known to be beneficial to both animals and humans, and there is currently debate about whether or not recommendations for their consumption should be given.
The difficulty in identifying the structure and role of biologically active metal ions is shown by biological chromium studies. Chromium is an excellent example of the significance of chemical speciation in biology: chromium (III) complexes have beneficial functions and low toxicity, while chromate, Cr (VI), is a human carcinogen. Since chromium (III) is widely recognised as an important trace metal, dietary reference intakes (DRIs) have been developed, and it is available as a dietary supplement. However, its role as a necessary component has recently been called into question. Some critical questions remain unanswered after sixty years of chromium research: I the structure of a biologically active chromium complex; (ii) methods for determining chromium status in humans; and (iii) elucidation of its exact mechanism of action in glucose and lipid metabolism. It's true that not being able to isolate a biologically active chromium complex does not appear to be a problem: absence of evidence does not imply absence. Since molybdenum and cobalt are found at similar low concentrations, the low level of chromium should not be a problem.
Biochemistry, the chemistry of life, is dependent on a large number of elements, according to a biological PSE that considers the two issues synonymous with the sense of "necessary" and distinguishes general from particular cases (Figure 2). The key characteristics of such a PSE are that I most non-metals are used (except noble gases); (ii) only B and Si from the metalloids (B, Si, Ge, As, Sb, Te) are known to have beneficial effects for humans and are important for certain species; and (iii) group 13 is unused (except for boron). A biological periodic table's individual elements have been thoroughly explored.
The basic elements are indicated by a biological periodic system of elements (PSE). With the exception of chromium (Cr), which is shown with an upward diagonal pattern (see text), the essential elements for most forms of life are shown in black, and the essential elements that are more limited for certain forms of life are shown in grey. The lanthanides and actinides (asterisk after lanthanum (La) and actinium (Ac) elements are not seen (Ac). The classes are numbered one through eighteen.
Given the renewed interest in the interesting and difficult chemistry of certain metals in special microbes, it's worth remembering the relative importance of transition metals and zinc in mammalian biology: Fe Zn > Cu > Mn > Mo (in the pterin cofactor) and Co (in the cofactor) (in the corrin cofactor). Around 5 g Fe, 2 g Zn, 100 mg Cu, 12–20 mg Mn, 5 mg Mo, and 2 mg Cr and Co are found in a 70 kg human.
Estimates of the number of metalloproteins containing zinc, iron, and copper were made by searching the genome database for signatures of metal binding sites in protein sequences. With such forecasts, a true understanding of these three metals at the systems level, i.e., their metalloproteomes, is beginning to emerge. An attempt is being made to annotate the functions of these metalloproteins based on structural similarity with proteins with recognised functions. Just four molybdoenzymes and two vitamin B12-dependent enzymes are identified in humans. However, there are no figures of how many enzymes use manganese as a cofactor.
We don't seem to have a comprehensive list of basic elements for any given species. The discovery of functions for elements like Cd and REE in metalloproteins (see above) suggests that our understanding of chemical element essentiality in various types of life is still incomplete. Metalloproteomes of microorganisms are still mostly uncharacterized, according to an "omics" report [16]. Then there's the question of what functions the other components have.
The Non-Essential Elements
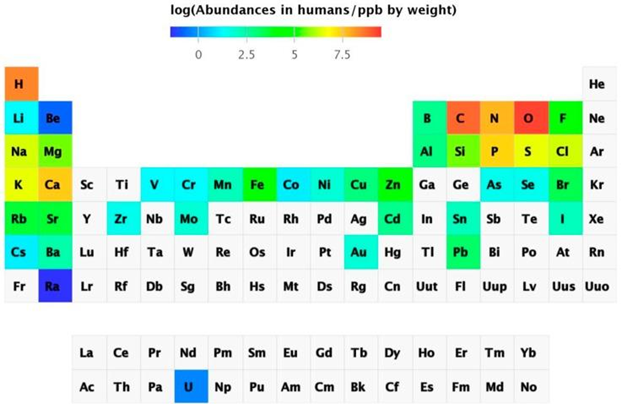
Metal determination using instrumental analytical chemistry has progressed dramatically. With sub-ppt detection limits, inductively coupled plasma mass spectrometry (ICP-MS) can detect virtually all naturally occurring elements in biological samples. It's also possible to detect radioactive uranium. As a result, salmon eggs contained 74 of the 78 components. The abundance of chemical elements in humans has been summarized in a periodic table.
Abundance of the chemical elements in humans
Non-essential elements are found in far higher concentrations than essential elements, which are present in the lowest concentration. Sr is present at ppm levels, and Rb, Ba, Ni, As, Al, and Ti (not shown in Figure 3) are present at ppb levels, although there are also others that are thought to have primarily toxic effects, such as Cd, Hg (not shown in Figure 3), and Pb, which are variable and dependent on exposure. Some components accumulate over time, while others are tightly regulated. The existence of non-essential elements following a skewed distribution based on exposure has been proposed as a criterion for elements being essential, namely homeostatic regulation for essential elements leading to a normal distribution but the presence of non-essential elements leading to a skewed distribution. Although certain elements are bioactive and have beneficial health effects, many non-essential elements are also bioactive. They are not chemically inert and can function as catalysts, pharmacologically active agents, or toxic substances. As a result, it's important to take a wider view of metal ions in biology, one that extends beyond the emphasis on vital metal ions to include the many elements that are present but whose functions are unknown. We don't know how animals and humans cope with these seemingly non-essential components, whether their existence indicates a lack of selectivity in absorption processes, whether there are any unique detoxification mechanisms, or what the exposure limits and biological effects are.
Complex interactions with non-essential elements and other nutrients are part of the delicate balance among the essential elements. Others are conditioned by too much (overload) or too little (deficiency) of one. Zinc supplementation, for example, can lead to copper deficiency, and a lack of an essential element can lead to the uptake of a non-essential element, which can be harmful. For example, in the case of an iron deficiency, cadmium uptake increases, interfering with the metabolism of essential elements like zinc.
Just concentrating on the most important aspects is insufficient. We need to keep an eye on the existence of non-essential elements, how their concentrations change, and how they influence biological system functions. We have some understanding of how defects or overload disrupt functions for the essential elements, though cause and effect in chronic diseases is often unclear. Effects at sub-toxic concentrations of non-essential elements are more difficult to determine. Since neurobiological parameters are adversely affected even at previously agreed blood lead levels, the Centers for Disease Control and Prevention reduced blood lead levels of concern for children aged 1–5 from 10 g/dL to 5 g/dL in 2012 to classify children and areas with lead-exposure hazards. Average normal levels in the US population are 1.6 µg/dL for adults and 1.9 µg/dL for children. Clearly, more solid research on how each individual element affects animals and humans is needed.
We're now starting to notice that there is a slew of inherited metal metabolism disorders in humans. Animals have been shown to have fewer differences than humans. Our understanding of how mutations in the many genes that encode metal regulatory processes influence metal toxicity and metal dose-responses quantitatively is extremely limited. Polymorphisms in the 12 human metallothionein genes mean that metal metabolism is affected. An Asn27Thr substitution in metallothionein-1A, for example, affects its zinc-binding ability and, when combined with polymorphisms in other human metallothionein genes, is linked to the development of type 2 diabetes, coronary heart disease, and other diabetes complications, as well as altered metabolism of toxic metal ions such as cadmium, lead, and mercury. Toxic metal levels in the blood are linked to polymorphisms in metal transporter genes and other genes, indicating that certain people are more vulnerable to the toxic effects of certain metal ions.
New applications and manufacturing processes expose animals and humans to a variety of metals they have never been exposed to before, especially those from the PSE's bottom half. Some exposures are amplified by food chains and food webs, which has mostly unexplored consequences for more recently used metal ions. REE are used in batteries, magnets, lighting, and optical fibres; gallium, indium, and tellurium are used in solar cells; hafnium and tantalum are used in optical devices; and ruthenium, rhodium, palladium, and their analogues osmium, iridium, and platinum are used in fuel cells. Another problem is the growing number of nanomaterials applications. They have different reactivities, which are also intensified. We're often exposed to metallodrugs for medicinal or medical purposes, and we use implants (Si), metal prostheses (Co), and dental fillings (Hg), all of which have practical implications for living systems. Increased use of additional elements brings a new dimension to exposure and contamination in air, soil, and water, with major consequences for health and aetiology and pathology research.
Most species have symbiotic relationships with others and must protect themselves from parasites. The gut contains a large number of commensal bacteria (the microbiome) that help with food digestion. Microorganisms use a different complement of metal ions than animals and humans, and they use metal ions that animals and humans do not seem to use. Many bacteria, including the ulcer-causing pathogen Helicobacter pyloris, need nickel. As a result, depriving this organism of nickel offers a targeted therapeutic approach for eradicating it from the upper digestive tract. Some trace element deficiencies can be caused by an impact on symbiotic species that need specific trace elements, rather than the host directly.
Conclusions
In the study of bio metals, chemistry and biology must work together. It is not sufficient to create only structures or to discover only functions. Sugars and lipids, for example, contain arsenic, but the presence of such structures does not prove that arsenic is needed.
We seem to be preoccupied with regulating energy intake from protein, fat, and sugar in the diet, but pay little attention to the intake of essential elements that allow metabolism, or the existence of apparently non-essential elements. Metals have become relatively easy to calculate with high sensitivity and precision thanks to modern instrumentation. Unlike the identification of millions of organic compounds to which we are exposed, which usually requires very complex and therefore costly methods of study, the number of metals to be screened is less than a hundred, and monitoring them is relatively inexpensive. Because of metal ions' high reactivity and catalytic ability, their levels in our diet and environment play an important role in our health, triggering disease or affecting its development, and influencing healthy ageing. It's still true in 2015 as it was in 1951: trace element functions in biology "might hold the answers to many unresolved biochemical and biological problems."
4.3.1 Na/K-pump:
The energy-intensive method of pumping molecules and ions through membranes "uphill" - against a concentration gradient - is known as active transport. A carrier protein is needed to transport these molecules against their concentration gradient. Carrier proteins can carry solutes along a concentration gradient (during passive transport), but some can also move solutes across a concentration gradient (from low to high concentration) with the addition of energy. Pumps are carrier proteins that are used to transfer materials toward their concentration gradient during active transport. ATP provides the energy for most active transport, just as it does for other forms of cellular activities. Transferring a phosphate group directly to a carrier protein is one way ATP drives active transport. This can cause the carrier protein to change shape, allowing the molecule or ion to cross the membrane to the other side. The sodium-potassium pump, shown in Figure below, is an example of an active transport system that exchanges sodium ions for potassium ions across the plasma membrane of animal cells.
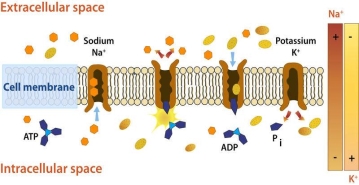
Sodium and potassium ions are moved by the sodium-potassium pump mechanism through broad concentration gradients. It pumps three sodium ions out of the cell and into the extracellular fluid, and it transfers two potassium ions into the cell where potassium levels are high.
Three sodium ions associate with the protein pump within the cell, as shown in Figure 1. The carrier protein then uses ATP to gain energy and change shape. It does so by expelling the three sodium ions from the cell. Two potassium ions from outside the cell bind to the protein pump at this stage. The potassium ions are then transported into the cell, where the cycle begins again. The sodium-potassium pump is located in almost every human cell's plasma membrane and is necessary for all cellular life. It controls cellular volume and helps to retain cell potential.
The Electrochemical Gradient
An electrical gradient form across the plasma membrane due to the active movement of ions across the membrane. Outside the cell, the number of positively charged ions exceeds the number of positively charged ions in the cytosol. As a result, the inside of the membrane has a comparatively negative charge, while the outside has a positive charge. The voltage across the membrane is caused by the charge difference. A separation of opposite charges, in this case through the membrane, causes voltage, which is electrical potential energy. Membrane potential is the voltage through a membrane. The conduction of electrical impulses along nerve cells is highly dependent on membrane potential.
The membrane potential favours the movement of positively charged ions (cations) into the cell and the movement of negatively charged ions (anions) out of the cell since the inside of the cell is negative relative to the outside. So, a chemical force (the ions' concentration gradient) and an electrical force (the influence of the membrane potential on the ions' movement) drive the diffusion of ions through the plasma membrane. An electrochemical gradient is the product of these two forces interacting, and it will be addressed in depth in the "Nerve Cells" and "Nerve Impulses" definitions.
4.3.2 Carbonic anhydrases:
Carbonic anhydrase is an enzyme that helps the conversion of carbon dioxide and water into carbonic acid, protons, and bicarbonate ions to occur quickly. This enzyme was first discovered in cow red blood cells in 1933. It has also been discovered in large quantities in all mammalian tissues, plants, algae, and bacteria. There are three types of this ancient enzyme (called alpha, beta and gamma carbonic anhydrase). Despite the fact that members of these various groups have little in common in terms of sequence or structure, they all serve the same role and need a zinc ion at the active site. Mammalian carbonic anhydrase is in the alpha class, plant enzymes are in the beta class, and the enzyme from methane-producing bacteria found in hot springs is in the gamma class. As a result, it's clear that these enzyme groups formed independently to produce a common active site. The alpha, beta, and gamma carbonic anhydrase enzymes are represented by PDB entries, which are shown from top to bottom. In these diagrams, the zinc ions in the active site are coloured blue. The alpha enzyme is a monomer, while the gamma enzyme is a trimeric complex. Despite the fact that this beta enzyme is a dimer, it has four zinc ions bound to it, suggesting four potential enzyme active sites. Other members of this class form tetramers, hexamers, or octamers, implying that dimer is most likely a building block.
Carbonic anhydrases in mammals exist in about ten slightly different ways, depending on the tissue or cellular compartment in which they are found. The sequence variations in these isozymes cause specific differences in their behaviour. As a result, isozymes present in certain muscle fibres have lower enzyme activity than salivary gland isozymes. While the majority of carbonic anhydrase isozymes are soluble and secreted, others are bound to epithelial cell membranes. Please visit the European Bioinformatics Institute's Protein of the Month feature for a more in-depth look at carbonic anhydrase from a genomic perspective.
Alpha (top), beta (middle), and gamma (bottom) carbonic anhydrases.
Carbonic Anhydrase in Health and Disease
Carbonic anhydrase is essential in the control of pH and fluid balance in different parts of our body because it generates and uses protons and bicarbonate ions. It aids in the secretion of acid in our stomach lining, as well as making pancreatic juices alkaline and our saliva acidic. The water content of the cells in our kidneys and eyes is influenced by the transport of protons and bicarbonate ions released in these organs. As a result, carbonic anhydrase isozymes perform various functions at different sites, and their absence or malfunction can result in a variety of diseases, ranging from a loss of acid production in the stomach to kidney failure.
When the fluid that keeps our eyes in shape builds up, it also pushes against the optic nerve in the eye, potentially damaging it. Glaucoma is the medical term for this disease. Inhibitors of carbonic anhydrase have been used to treat glaucoma in recent years. By inhibiting this enzyme, the fluid equilibrium in the patient's eyes is shifted, reducing fluid build-up and relieving pressure. One such inhibitor (a sulfonamide) is bound to human carbonic anhydrase in the structure of PDB entry, which is coloured green in the figure (isozyme II). This inhibitor binds near the active site and interferes with the interactions of the water bound to the zinc ion, preventing the enzyme from working. Unfortunately, prolonged use of this drug can cause side effects such as kidney and liver damage by affecting the same enzyme found in other tissues.
Inhibitor (green) bound in the active site of carbonic anhydrase.
Carboxypeptidase and Substrate Binding
Proteins are important molecules in organisms which serve a variety of roles throughout the human body, from supplying tensile strength to bones and tendons to storing and transporting essential substances like oxygen and iron in the body. As a result, proteins from foods must first be segregated into their constituent amino acids within the body's cells. The amino acids are then used to construct the proteins that our bodies need.
A hydrolysis reaction is used by the cell to break down a protein into its constituent amino acids. The protein binds to a water molecule, forming an amino acid and a new protein. The pancreas secretes the enzyme carboxypeptidase A, which is used to speed up the hydrolysis reaction. This enzyme is made up of a single 307-amino-acid chain, as shown in Figure 2. It takes on a compact, globular shape that contains helices and pleated sheet regions. This globular form has a pocket-like region where a substrate can be placed. The enzyme's active site is located here.
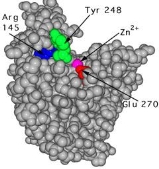
Since the active site changes significantly when the substrate binds, carboxypeptidase A is a clear example of the induced-fit principle. Figures 2 and 3 depict the carboxylase protein with and without a bound substrate. When the active site is complexed with a substrate, it changes shape. The active site of carboxypeptidase closes in around the protein substrate as it binds to it. If the terminal residue has an aromatic or bulky hydrocarbon side chain, hydrolysis of the peptide bond is more likely. At the active site, a zinc ion (Zn2+) is closely bound and aids in catalysis. For the enzyme to recognise the terminal amino acid in the peptide chain, three hydrogen bonding and electrostatic interactions are needed. Interactions with Zn2+ and the carboxypeptidase molecule stabilise the intermediate. Proton transfer and peptide bond cleavage are the final steps. This whole process necessitates a high level of mobility in the carboxypeptidase A protein.
The unbound carboxypeptidase. An enzyme is described by this molecular model. The cpk, or space-filled, representation of atoms is used to display the active site's estimated volume and form.
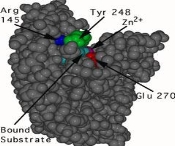
This is a cpk representation of carboxypeptidase A in which the active site has a substrate (turquoise) bound to it. The active site is in a conformation that has been caused. To show the shape shift, the same three amino acids (Arg 145, Tyr 248, and Glu 270) are numbered.
4.4.1 Trace Elements
Trace elements (or trace metals) are minerals found in trace quantities in living tissues. Some are believed to be nutritionally important, others may be essential (though the evidence is only suggestive or incomplete), and the rest are deemed unnecessary. Trace elements are mainly used as catalysts in enzyme systems; however, certain metallic ions, such as iron and copper, are involved in energy metabolism's oxidation-reduction reactions. Iron, which is found in haemoglobin and myoglobin, is also essential for oxygen transport.
If ingested at high enough levels for long enough periods of time, all trace elements are toxic. For certain critical trace elements, the difference between toxic and ideal intakes to fulfil physiological needs is important, but for others, it is much smaller.
This chapter provides an overview of the roles of iron, zinc, fluoride, selenium, copper, chromium, iodine, manganese, and molybdenum in the aetiology and prevention of chronic diseases. Aluminum, cadmium, mercury, arsenic, and lead are also discussed; these elements have not been shown to be important for humans, but the committee considered them because they are commonly consumed as pollutants in food or water. Interactions among the various trace elements are briefly discussed as well.
Epidemiologic evidence on the connection between several trace elements and the occurrence of diseases like cancer, cardiovascular disease, and hypertension is lacking. Cadmium, chromium, and selenium have been the subjects of the majority of these tests. Furthermore, the majority of the literature does not pertain to dietary exposure, but rather to inhalation exposure in the workplace, for example. Animal feeding trials have also yielded inconclusive results. The committee discusses information gaps and recommends research directions.
4.4.2 Evidence Associating Trace Elements with Chronic Diseases
IronIron is found in every cell of the body. It serves as an oxygen carrier in the blood and muscles as a part of haemoglobin and myoglobin. Women in their reproductive years need more iron than men due to iron losses during menstruation. As a result, the Recommended Dietary Allowance (RDA) for women aged 11 to 50 is 18 mg per day, but only 10 mg per day for men aged 19 and up. Women have a harder time maintaining this high intake because they have a lower caloric intake, and the typical American diet only contains 6 to 7 mg of iron per 1,000 calories. Due to the increased need for iron during times of rapid development, children from infancy to puberty, as well as pregnant women, may not consume enough iron to meet their needs.
Many factors influence iron absorption. Heme iron, which is found in meats, poultry, and fish, is more easily absorbed than nonheme iron, which is found in both plant and animal foods. Nonheme iron absorption is aided by ascorbic acid, but dietary fibre, phytates, and certain trace elements can reduce it. The efficiency with which the body absorbs iron from a given food is not indicated by food composition data. The National Research Council's publication Recommended Dietary Allowances (NRC, 1980) explains how to quantify usable iron.
Since 1909, the abundance of iron in food has increased, owing primarily to the enrichment of flour and cereal products. According to the 1977-1978 Nationwide Food Consumption Survey (NFCS), respondents of both sexes aged 1 to 18 years old and females aged 19 to 64 years old failed to reach their RDA for iron on average (USDA, 1984). These results are supported by the Continuing Survey of Food Intakes of Individuals (CSFII), which was conducted in 1985 and 1986 (USDA, 1987a, b). Failure to reach the RDA, on the other hand, is not a reliable predictor of iron deficiency.
A professional scientific group of the Federation of American Societies for Experimental Biology (FASEB) assessed iron status using data from the National Health and Nutrition Examination Survey (NHANES II), which was conducted from 1976 to 1980. (LSRO, 1984a). The assessment used five metrics from three different models. Children 1 to 2 years old, males 11 to 14 years old, and females 25 to 44 years old all had a moderately high prevalence of iron deficiency. Children 1 to 5 years old and females 25 to 54 years old had the worst iron status for those with incomes below the poverty line (LSRO, 1984a).
CancerEpidemiologic and Clinical Studies
Iron deficiency is a risk factor for the Plummer-Vinson (Paterson-Kelly) syndrome, which was once widespread in parts of Sweden but is now almost completely eradicated thanks to better nutritional status, especially in terms of dietary iron and vitamins (Larsson et al., 1975; Wynder et al., 1957). This disorder has been linked to an increased risk of cancers of the upper digestive tract, especially cancers of the oesophagus and stomach, indicating that underlying iron deficiency may be one of the factors contributing to the development of these cancers. Low dietary iron intake, on the other hand, has not been linked to cancers at these sites in epidemiologic studies (Schottenfeld and Fraumeni, 1982).
Boing et al. (1985) found a positive relationship between estimated iron consumption and mortality from colorectal and pancreatic cancer in men and gallbladder cancer in women in a correlation study of diet survey data and cancer mortality rates for 11 regions of the Federal Republic of Germany. Ferritin levels were significantly higher in men over 50 who developed cancer, particularly primary hepatocellular carcinoma (PHC), than in controls without cancer in a prospective cohort of 21,513 Chinese men in Taiwan, whereas serum transferrin levels were lower in men who developed cancer (excluding PHC) (Stevens et al., 1986). While iron stores were not specifically measured, these findings most likely represent a connection between increased cancer risk and increased body iron stores.
In hematite miners and foundry employees, occupational inhalation exposure to iron oxides has been linked to an increased risk of lung cancer (Kazantzis, 1981), but there were also other carcinogens present, such as ionising radiation, polycyclic aromatic hydrocarbons (PAHs), and cigarette smoke. As a result, the increased cancer risk cannot be due to iron alone (Doll, 1981; Kazantzis, 1981).
Patients with idiopathic hemochromatosis, a disease characterised by abnormal iron accumulation in the liver and sometimes cirrhosis, have a significantly increased risk of hepatocellular carcinoma, according to clinical reports (Ammann et al., 1980; Bomford and Williams, 1976; Strohmeyer et al., 1988).
Overall, these human studies do not show that iron exposure, whether by diet or other means, plays a role in the aetiology of human cancer.
Animal Studies
Compared to 6 months in an iron-sufficient population, iron-deficient rats given 1,2-dimethylhydrazine (DMH) formed neoplastic liver lesions in 4 months (Vitale et al., 1978). The lack of iron tended to encourage carcinogenesis, according to the scientists.
In BALB/c mice with transplanted Merwin Plasma Cell-II tumours, the impact of iron deficiency on tumour growth and host survival was investigated (Benbassat et al., 1981). In weanling mice, an iron deficiency slowed body development and tumour growth, but not in adults. The cause of this disparity in response has yet to be identified. In iron-deficient female Wistar rats, mammary tumours induced by intragastric administration of dimethylbenz[a]anthracene (DMBA) and fibrosarcomas induced by subcutaneous injections of methylcholanthrene (MCA) were examined (Webster, 1981). In comparison to controls, there was no difference in induction time, tumour location, total number of tumours, or metastasis incidence in iron-deficient rats. Iron deficiency did not appear to prevent carcinogenesis in this study, as it did in the study by Benbassat et al (1981). Later, albino iron-deficient rats were painted with the oral carcinogen 4NQO and left untreated in another study (Prime et al., 1986). There was no difference in tumour growth or epithelial dysplasia between the iron-deficient and iron-sufficient species, according to these researchers.
The effects of a cocarcinogen, ferric oxide, on the metabolism of benzo[a]pyrene (BaP) was investigated using isolated perfused rabbit lung (Warshawsky et al., 1984). According to the findings, ferric oxide increased BaP's production of dihydrodiols, which could then be metabolised to the ultimate carcinogenic types. DBA-2 mice given supplementary iron (24 mg/kg body weight) and inoculated with L1210 cells produced more tumour cells than controls over time. Animals inoculated with L1210 cells and given higher doses of supplementary iron (250 mg/kg body weight) died earlier than untreated but inoculated controls (Bergeron et al., 1985). High doses of supplemental iron, the authors concluded, can increase neoplastic proliferation or metastasis in vivo. Iron deficiency has been linked to an increased risk of cancer in animals on many occasions (Weinberg, 1983).
Animal research, in comparison to epidemiologic studies, show that iron can either stimulate or inhibit tumour growth depending on the circumstances. The effects of iron deficiency on tumour growth are controversial, and some iron compounds can act as cocarcinogens. The method of administration, the dosage, and the type of iron compound all seem to have an effect on the outcome.
Short-Term Tests
With and without metabolic activation by the S9 fraction, Brusick et al. (1976) discovered that Fe [II] as iron sulphate caused reverse mutations in Salmonella typhimurium strains TA1537 and TA1538. Another research looked at 45 metal salts to see whether they could cause morphological changes in Syrian hamster foetal cells in vivo. Positive transformation assays for iron were among the trace elements for which positive transformation assays were obtained (DiPaolo and Casto, 1979).
4.4.3 Coronary Heart Disease
Epidemiologic Studies
Iron deficiency is more common in women than in men, which has been suggested as an explanation for premenopausal women's lower coronary heart disease (CHD) incidence (Sullivan, 1986); however, no epidemiologic evidence supports this theory.
Iron-Deficiency AnaemiaEpidemiologic Studies
Iron deficiency anaemia occurs when the amount of iron in the body falls short of what is needed for the normal formation of haemoglobin, iron enzymes, and other iron compounds. It is the world's most common nutritional deficiency (Dallman et al., 1979) and the leading cause of anaemia in Western countries. However, in the United States, overall prevalence is poor. NHANES II showed that the highest prevalence (9.3%) occurred among children 1 to 2 years old; next came women age 15 to 19 (7.2%) and 20 to 44 (6.3%). Men aged 15 to 64 years old had a prevalence rate of less than 1%. (LSRO, 1984a).
Iron deficiency anaemia is most often caused by a lack of iron in babies and small children, as well as blood loss or pregnancy in adults. Infections and chronic illnesses, not iron deficiency, are the most common causes of anaemia in the elderly. The prevalence of iron deficiency anaemia varies greatly depending on the diagnostic criteria used (Charlton and Bothwell, 1982; Reeves et al., 1983), and it can be influenced by physiological, pathological, and dietary causes.
With rises in caloric intake from fats and refined sugar, the amount of dietary iron has decreased in certain parts of the population. Iron consumption has decreased in tandem with caloric intake. Iron deficiency anaemia, for example, is more common in women than in men, even though there is no pathological blood loss. Women consume less food and hence ingest less iron, but their needs for iron are higher due to the loss of iron during menstruation. Iron supplementation, fortification, and dietary changes are examples of preventive measures. For example, increasing intake of nutrients and foods that promote iron absorption (e.g., vitamin C, meat, and fish) or reducing intake of foods that inhibit iron absorption are examples of preventive measures (e.g., phytates).
The determination of the plasma iron level and iron-binding potential are two older but still effective methods for assessing body iron stocks. A more precise measurement of iron stocks is obtained by measuring serum ferritin and free erythrocyte protoporphyrin concentrations.
Animal Studies
The hearts of rats with experimentally induced iron deficiency anaemia showed dramatic morphological changes. Cellular hypertrophy, as well as cellular degeneration and interstitial fibrosis, were prominent features of these shifts (Rossi and Carillo, 1983). Rossi and colleagues used reserpine to treat iron-deficient anaemic rats in other trials (Rossi and Carillo, 1982; Rossi et al., 1981). Cardiac hypertrophy was observed in the hearts of anaemic rats who were not given reserpine, as shown by increases in heart weight and muscle cell level. The hearts of animals given reserpine did not enlarge. The researchers hypothesised that noradrenaline can play a role in iron-deficiency anaemia-induced cardiac hypertrophy.
Zinc
Zinc plays an important role in nucleic acid metabolism, cell replication, tissue repair, and growth through its action in nucleic acid polymerases, which is found in over 200 enzymes. The potentially rate-limiting enzymes involved in DNA synthesis are among the zinc-dependent enzymes. Zinc also has a number of well-known and biologically significant interactions with hormones, and it plays a role in hormone synthesis, storage, and secretion. In the United States, serious, moderate, and minor zinc deficiencies have been identified (Hambidge et al., 1986).
Shellfish (especially oysters), beef, and other red meats are the best sources of zinc. Poultry, eggs, hard cheeses, milk, yoghurt, legumes, nuts, and whole-grain cereals are all healthy sources of this nutrient. Zinc absorption may be hampered by a variety of dietary factors, including other minerals, phytates, and dietary fibre (Hambidge et al., 1986). At the turn of the century, zinc has been used in a variety of foods. Until the middle of the 1930s, people got almost equivalent quantities of zinc from animal and plant foods, but since 1960, animal foods have accounted for roughly 70% of the food supply zinc. Zinc from animal sources tends to be more readily absorbed than zinc obtained from plants.
Zinc RDA for people 11 years and older is 15 mg per day (NRC, 1980), but zinc available in the food supply is just 12.3 mg per capita (see Table 3-5). Only since 1984 have national surveys been used to estimate zinc consumption. Men and women aged 19 to 50 consumed an average of 94 and 56 percent of their RDA, respectively, according to the 1985 NFCS, while children aged 1 to 5 consumed 73 percent of their RDA of 10 mg/day (USDA 1986, 1987b).
A FASEB group (LSRO, 1984b) evaluated NHANES II data and concluded that serum zinc levels are insufficient for assessing zinc nutritional status in individuals, but that low values may help in identifying groups whose zinc status should be investigated further.Atherosclerotic Cardiovascular DiseasesEpidemiologic and Clinical Studies
Klevay (1975) hypothesised that an excess of zinc relative to copper may underpin CHD based on knowledge of the relationships between zinc and copper and several risk factors for CHD, including elevated serum cholesterol and hypertension. For example, adding more than 10 times the RDA of zinc to the diets of 12 adult men for 5 weeks while maintaining normal copper levels resulted in a substantial decrease in high-density lipoprotein (HDL) cholesterol but no improvement in total cholesterol (Hooper et al., 1980). This theory, as well as the contradictory evidence supporting it, is explored in the copper section.
Animal Studies
There were no animal studies on the connection between zinc and cardiovascular disease that the committee could find.CancerEpidemiologic and Clinical Studies
There have been few epidemiologic studies looking into the link between zinc exposure, especially dietary zinc, and cancer risk. Zinc levels in soil, food, and blood have all been linked to a variety of cancers in association studies (Schrauzer et al., 1977a, b; Stocks and Davies, 1964).
Blood and hair samples from a random sample of 58 men and 53 women who had undergone esophagoscopy with biopsy were tested for zinc, riboflavin, and vitamin A components in an attempt to classify etiologic factors for esophageal cancer in the very-high-risk region of Linxian in Hunan Province, China (Thurnham et al., 1982). Zinc levels in plasma and hair did not vary substantially between subjects with normal histology and those with lesions that were thought to be cancer precursors (e.g., esophagitis, dysplasia, acanthosis). Another analysis of a similar random sample from a low-risk region in Shandong Province showed no variations in zinc levels in blood and hair (Thurnham et al., 1985).
Just one case-control cancer study looked at dietary zinc. Kolonel et al. (1988) discovered that patients with prostate cancer aged 70 and up consumed more zinc (from supplements rather than food) prior to the onset of the disease than matched population controls. Occupational studies of workers who were exposed to zinc by inhalation (usually in the presence of other trace elements including copper, lead, arsenic, and chromium) have found no evidence that zinc is a cancer risk factor (Gerhardsson et al., 1986).
Zinc levels in cancer patients' serum or tissue have been compared to those in controls in a number of clinical trials. Sample sizes were small in most of these studies, controls were not well suited to the cases, even on age, and possible confounding factors were not taken into account in the analyses. The findings have been a bit of a mixed bag. Researchers have found lower serum zinc levels in patients (Atukorala et al., 1979; Davies et al., 1968; Lin et al., 1977; Mellow et al., 1983; Sharma et al., 1984; Whelan et al., 1983) and higher zinc levels in patients (Adler et al., 1981) in studies of cancer patients at many different sites. In other trials, no variations between patients and controls were discovered (Feustel and Wennrich, 1986; Manousos et al., 1981; Smith et al., 1971; Strain et al., 1972; Thurnham et al., 1982). Serum copper levels were also tested in many of these trials, and they were consistently higher in the patients than in the controls, resulting in lower zinc-to-copper ratios. The observation of Sharma et al. (1984), who confirmed that depressed serum zinc levels in lymphoma patients returned to normal following chemotherapy, suggests that the zinc results in these studies were a result rather than a cause of the cancers.
Since the prostate contains the highest concentrations of zinc in the body, it is a focus of research on zinc and cancer (Hambidge et al., 1986). Researchers compared zinc levels in prostate tissue from healthy subjects and patients with benign prostatic hypertrophy (BPH) and cancer in several studies. Feustel and Wennrich (1984); Feustel et al. (1982); Györkey et al. (1967) found that zinc concentrations were lowest in carcinomatous tissue, maximum in BPH tissue, and intermediate in normal tissue.
Zinc has an antagonistic relationship with copper and interacts with other trace elements (Mertz, 1982). (See discussion below under Zinc-Copper Interactions). Zinc interacts with vitamin A as well (Solomons and Russell, 1980). As a result, human studies on zinc may reveal fundamental relationships between other nutrients and the diseases of interest. Human research, on the other hand, show no evidence that zinc intake plays a significant role in cancer aetiology.
Animal Studies
Zinc has been shown to have both stimulating and inhibiting effects on tumour growth in animals, according to research. Several studies have shown that a zinc-deficient diet slows the development of transplanted tumours and increases survival time (Barr and Harris, 1973; Beach et al., 1981; DeWys and Pories, 1972; DeWys et al., 1970; Fenton et al., 1980; Mills et al., 1984; Minkel et al., 1979). These results indicate that tumour cells that are rapidly growing need zinc to develop. Severe zinc deficiency, with or without concomitant malignancies, is lethal in and of itself, so it is not recommended as a therapeutic modality.
In comparison to transplanted tumours, zinc deficiency tends to promote chemically induced carcinogenesis. Gabrial et al. (1982) found that in zinc-deficient rats, the frequency of esophageal tumours caused by nitrosomethylbenzylamine was much higher than in control rats. In other research, high zinc intake suppresses the carcinogenesis of dimethylbenzylamine in Syrian hamsters (Poswillo and Cohen, 1971) and azo dyes in rats (Poswillo and Cohen, 1971). (Duncan and Dreosti, 1975). Large concentrations of zinc (200 mg/ litre) in the drinking water of C3H mice, on the other hand, counteracted the protective effect of selenium against spontaneous mammary carcinoma and resulted in a significant increase in tumour development, according to Schrauzer (1979).
Beach et al. (1981) elucidated the two distinct effects of zinc deficiency on carcinogenesis in BALB/c mice fed diets containing four levels of zinc starting at 6, 3, 1, and 0 weeks before injection of Moloney sarcoma virus (MSV). Mice fed marginally and moderately zinc-deficient diets developed more sarcomas than control mice, while mice fed a highly zinc-deficient diet developed fewer and smaller sarcomas. Low-zinc diets were fed for 6 weeks before MSV injection, which resulted in less sarcomas being triggered and tumour progression being slowed. Mice had a longer tumour latency and a faster tumour regression period after a reasonable magnitude and length of zinc deprivation.
These seemingly conflicting effects of zinc deficiency—tumour growth enhancement at some levels and tumour growth suppression at others—could mean that two distinct mechanisms of action are at work (Beach et al., 1981). Zinc deficiency has been shown to affect many aspects of immunocompetence in both laboratory animals and humans (Beach et al., 1979; Fernandes et al., 1979). (Golden et al., 1978). As a result, changes in host immunologic function caused by zinc deficiency may lead to changes in host-tumour interactions. However, zinc is known to affect many aspects of host and tumour metabolism, including nucleic acid and protein synthesis, and zinc deficiency has the greatest impact on tissues in rapid development (Hurley, 1981). As a result, zinc is required for tumour development. In zinc-deficient species, altered DNA synthesis by neoplastic tissue leads to tumour growth inhibition. In pregnant and foetal rats, zinc deficiency induces chromosome aberrations (Bell et al., 1975).
The relative functions of host immune tolerance and the relationship of host and tumour metabolism are difficult to determine. Nonetheless, it appears that the effect of zinc nutrition on carcinogenesis is influenced by the interaction of the host and tumour, at least in part. In comparison to human studies, animal studies show that zinc can either promote or inhibit tumour development, possibly through its effects on host immunocompetence or nucleic acid synthesis, as mentioned above.
4.4.5 Toxic effect of metals:
Two Classes of Toxic Metal Compounds
Excess quantities of an important metal may be just as harmful as insufficient amounts, as stated in the previous section. This condition may occur as a result of inadvertently ingesting the element or from metabolic disorders that render normal biochemical mechanisms that regulate uptake and distribution ineffective. One form of metal toxicity is represented by these possibilities. The entry of non-essential metals into the cell through food, skin absorption, or respiration is the other large category. Because of the public health threats posed by chemical and radio isotopic environmental contaminants, the toxicities associated with this latter class have gained a lot of attention recently.
In this segment, we look at examples from both categories and explore how bioinorganic chemistry can help with toxic metal removal and normal function restoration. Chelation therapy is one method, in which metal-specific chelating agents are given as drugs to help complex and excrete the unwanted excess element. One example of this method is the use of desferrioxamine to treat iron poisoning. A second role of bioinorganic chemistry is to recognise basic biological mechanisms that govern metal detoxification and to apply the principles discovered to help monitor the toxic effects of metal ions in the environment. Recent research on mercury tolerance and detoxification in bacteria is an excellent example of how biochemistry and molecular biology can be combined to uncover molecular events. This research, which led to the discovery of metalloregulatory proteins, is detailed in Section III.F below. It serves as a yardstick by which other studies into the processes of metal detoxification can be measured.
Copper Overload and Wilson's Disease
Wilson's disease is caused by a genetically inherited metabolic deficiency in which normal levels of copper are no longer tolerated. Liver disease, brain injury, and brown or green (Kayser-Fleischer) rings in the cornea of the eyes are the clinical symptoms. Wilson's disease patients have low levels of ceruloplasmin, a copper-storage protein; the gene and gene products responsible for the altered metabolism have yet to be identified. Chelation therapy, which uses K2Ca (EDTA) to replenish body calcium reserves depleted by EDTA coordination, 2,3-dimercaptopropan-1-ol (BAL, British Anti-Lewisite), or d-penicillamine to remove excess copper, eliminates symptoms. Copper is presumably removed as Cu(I) thiolate complexes by the sulfhydryl groups of the latter two compounds. Wilson's disease provides a great opportunity for modern methodologies to isolate and clone the gene responsible for the altered Cu metabolism, resulting in a rational treatment basis.
Iron Toxicity
Iron overload is also treated with chelation therapy. Acute iron poisoning, such as that caused by the inadvertent ingestion of FeSO4 tablets, causes gastrointestinal tract corrosion. Hemochromatosis, or chronic iron poisoning, is caused by the digestion of too much iron, which is normally supplied by cooking vessels. Siderosis is a condition caused by the consumption of large amounts of beer brewed in iron pots by members of the Bantu tribe in South Africa, who develop deposits of iron in their liver, kidney, and heart, resulting in organ failure. The siderophore desferrioxamine, a polypeptide with a very high affinity for Fe (III) but not for other metals, is the chelating agent of choice for iron toxicity. Ferrioxamine chelates are iron-transporting agents found naturally in bacteria. An active field of bioinorganic research is attempting to imitate and enhance natural systems in order to provide better ligands for chelation therapy (see Chapter 1).
Toxic Effects of Other Essential Metals
Most of the other metals mentioned in Table 9.1 are toxic when present in concentrations above their natural cellular levels. Vitamin D and parathyroid hormones regulate calcium levels in the body. Failure to control Ca2+ results in tissue calcification, stone formation, and cataract formation, a complex mechanism of which little is known (see Chapter 3). Chronic manganese poisoning, which may occur after inhaling metal-oxide particles, as in the case of Chilean miners, causes neurological symptoms that are close to Parkinson's disease. There has been evidence of neuronal injury. While Zn toxicity is uncommon, it can cause deficiencies in other important metals including calcium, iron, and copper. Cobalt poisoning can cause gastrointestinal problems as well as heart failure. Chelating agents, most commonly CaNaiEDTA, have been used to treat metal poisoning caused by certain elements, but the selectivity provided by the ferrioxamine class of ligands available for iron has not even been approached. Fortunately, these metals are only used in a few instances.
Plutonium: A Consequence of the Nuclear Age
Some of the chelating agents that were created to treat iron toxicity are now being used to treat plutonium poisoning. Salts and siderophores of diethylenetriaminepentaacetic acid (DTPA) are particularly effective. By tailoring the ligand to fully encapsulate the eight-coordinate Pu (IV) nucleus, some improvement over naturally occurring chelates has been made. While only a few people have been affected, ingestion of 239Pu, for example, as tiny particles of PuO2 at nuclear power plants, can be fatal. 239Pu releases high-energy ex particles, which cause cancers of the bone, liver, lung, and lymph nodes, to which transferrin transports it. Plutonium is one of the most toxic metals known, with a maximum tolerated dose of just 1.5 g. Now we'll look at some other, more traditional examples of industrial contaminants.
Mercury Toxicity and Bacterial Resistance
Weathering of mercury's most common ore, HgS, red cinnabar, releases Hg (II) ions into the atmosphere. Organomercurials of the general formula RHgX, which are used in agriculture, have also ended up as radioactive waste in the forest. RHgX and HgX2 bind to sulfhydryl groups in proteins with a high affinity, causing neurological disease and kidney failure. Metallothionein is a common protein target for reducing mercury toxicity. In Minimata, Japan, 52 people died after consuming mercury-contaminated fish and crustaceans near a factory waste outlet in 1953, in a widely publicised situation. While mercury in its volatile, elemental form, Hg (0), is said to be nontoxic, its conversion to alkylmercury compounds by anaerobic microorganisms using a vitamin B-12 biosynthetic pathway poses a serious health risk.
Mercury poisoning is treated with BAL, which has a high affinity for sulfur-donor ligands; N-acetyl penicillamine has also been suggested. Recently, a fascinating natural detoxification mechanism in mercury-resistant bacteria was discovered; once fully understood, this system may offer valuable techniques for treating heavy-metal poisoning in humans.
Bacteria have established mechanisms of tolerance to HgX2 and RHgX compounds, in which mercury is recycled back to Hg, presumably as a result of environmental strain (0). The bacterial mercury-resistance process involves at least five gene products. The basic absorption of mercury compounds is mediated by MerT and MerP. Two of the reactions, given in Equations (9.1) and (9.2), are catalysed by MerB, organomercury lyase, and MerA, mercuric reductase (9.2). The genes for these two proteins have been isolated from plasmids. The mer operon's usual gene arrangement
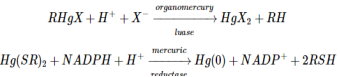
MerR, a metalloregulatory protein that regulates mer gene transcription, is a gene product. The MerR protein binds to DNA as a repressor in the absence of Hg (II), preventing transcription of the merT, P, A, and B genes and negatively autoregulating its own synthesis (Figure 9.1). When Hg (II) is present, these genes' transcription is activated. Interestingly, whether acting as an activator in the presence of Hg (II) or as a repressor in the absence of it, the MerR protein remains bound to the same site on DNA. Several cysteine residues in the carboxyl terminal region of the protein have been implicated as candidates for the mercury-binding site in random and site-specific mutagenesis experiments.
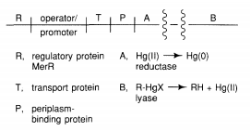
The merB gene encodes organomercury lyase, which performs the remarkable enzymatic step of breaking Hg-C bonds (Equation 9.1). It's a 22-kDa protein that doesn't contain any metals or cofactors. This chemistry is thought to be influenced by two cysteine-sulfhydryl groups on the protein, as seen in Equation 1. (9.3). Stereochemical analyses of the Hg-C bond cleavage showed that the structure is retained, suggesting that the Hg-C bond is not cleaved through a radical pathway. It has been proposed that a novel concerted SE2 mechanism exists. While sluggish, the enzyme turnover rates, which range from 1 minute for CH3HgCl to 240 minutes for butenyl mercuric chloride, are 105-108 times faster than the nonenzymatic rate.
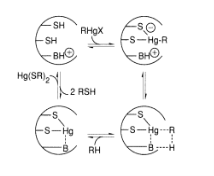
The FAD-containing merA gene product, mercuric ion reductase, has several pairs of conserved cysteines. Cysteine residues in the sequence 134-Thr-Cys-Val-Asn-Val-Gly-Cys-140 are known to contain a redox-active disulfide ring, and a redox-inactive pair of cysteines near the carboxyl terminus is also needed for the selective reduction of Hg, according to site-specific mutagenesis studies (II). The exact mechanism by which the enzyme achieves the chemistry shown in Equation (9.2) is unknown, but the flavin and disulfide/thiol centres' redox activities are certainly involved. This enzyme is responsible for both the complete clearance of Hg2+ provided by the MerB protein from RHgX compounds and the detoxification of mercury supplied directly from the environment as Hg (II) salts. In this fascinating class of Hg-resistant bacteria, Nature has clearly devised a remarkable mechanism for mercury detoxification.
Cadmium and Lead Toxicity
Acute or chronic exposure to these heavy metals can cause gastrointestinal, neurological, and kidney toxicity, among other symptoms. The use of unleaded fuel and the elimination of lead-containing pigments from paint has reduced the amount of lead released into the atmosphere each year significantly. Alkaline batteries, pigments, and plating are all sources of cadmium. Chelation therapy with CaNa2(EDTA) (acute) or penicillamine (chronic) can be used to treat lead poisoning (chronic). While both Cd (II) and Pb (II) bind to thionein's sulfhydryl groups, we know nothing about the molecular mechanisms through which these elements cause toxicity.
Metals as Carcinogens
While most metal ions have been found to be carcinogenic, Ni, Cr, and, to a lesser degree, Cd are the three most potent cancer-causing metals. Many nickel-containing ores contain nickel subsulfide, Ni2S3, which has been extensively studied and shown to be carcinogenic in humans and other species. In vitro gene replication infidelity was increased, and bacterial DNA repair was altered, according to short-term bioassays that included mutagenesis. The chromate ion (CrO42-), which enters cells through the sulphate uptake pathway and is eventually reduced to Cr (III) via a Cr(V)-glutathione intermediate species, is the most carcinogenic type of chromium. The latter complex binds to DNA, resulting in a kinetically inert but potentially harmful lesion. Despite the abundance of knowledge regarding metal-DNA interactions, the molecular mechanisms of metal-induced carcinogenesis remain unknown. Tumour initiation and tumour growth are two facets of the issue that are likely to involve different pathways. Bioinorganic chemists should be able to unravel knowledge of how metals function as carcinogens and mutagens as new techniques for studying the molecular events responsible for cancer (oncogenesis) become available. Metal/nucleic-acid chemistry is likely to play a role in such pathways, given that cancer has genetic origins. Metal-DNA interactions are an integral part of the antitumor drug process of cis- [Pt (NH3)2Cl2], as discussed later.
4.5.1 Minerals toxicity:
What is Mineral Toxicity?
Plants work in a similar manner. In reality, the entire universe operates on a delicate balance, and every living being in that world is bound by the law of balance. The precise proportions of the seven important micronutrients must be preserved. This leads to the cautious inference that too much leads to toxicity, while too little leads to deficiency, as previously mentioned.
Electron carriers, enzyme activation, providing osmoticum for turgor and development, maintaining charge balance, structural components, and more are all functions of mineral elements in plants.
Effects of Mineral Toxicity
Plants with mineral nutrient deficiencies experience stunting, abnormal thickening, and darkening of roots, reduced growth, massive disruption of cell and cell walls, reduced branching, small changes in the pH of the cytosol, an inability of an enzyme to align correctly with a reactant, iron chlorosis, oxidative stress, chlorosis, destruction of chloroplasts, and iron chlorosis.
This begs the question of how this delicate balance or precise proportion is achieved. There is a simple solution to this; the concentration of the mineral ion in tissue reduces the dry weight of tissues by 10% and is considered harmful. Since each plant has different nutritional needs, weights, energy requirements, and other factors, these concentrations vary between plants.
Another factor to remember is that an excessive amount of one element prevents the absorption of another. The presence of manganese toxicity, for example, is shown by the existence of brown spots surrounded by chlorotic veins. Manganese competes with magnesium and iron for absorption in this region, as well as preventing calcium translocation in the plant's shoot apex. As a result, manganese deficiency in plants causes a deficiency in iron, copper, and calcium.
Carcinogenic agents have the ability to destroy the genome or disrupt the cells that participate in the metabolic process. Many radioactive compounds are thought to be carcinogenic, but their carcinogenic properties are caused by the radiations they emit. Carcinogenic agents include gamma rays and alpha particles. Tobacco smoke, some dioxins, and inhaled asbestos are examples of non-radioactive carcinogens. Tobacco smoke emits toxic gases such as carbon monoxide, which can cause cancer. Carcinogenic compounds are often assumed to be synthetic chemicals, but they may be natural or synthetic. Carcinogenic compounds do not have to be harmful right away; they are sneaky.
4.5.2 Carcinogenic Substances
Cancer is a term used to describe a group of diseases that cause abnormal cell growth and have the potential to spread to other areas of the body. It is a disease in which the body's cells are weakened. Carcinogenic agents raise the risk of cancer by causing damage to the body's metabolic cells. They often cause damage to the cell's DNA, which is linked to a variety of biological processes in the body. Tumors result as a result of this.
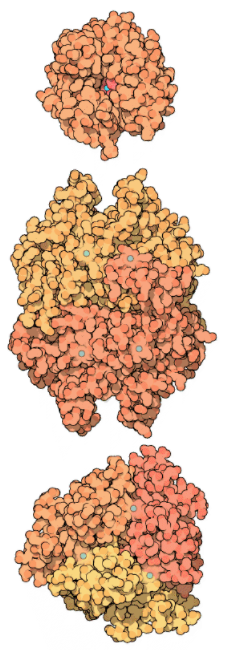
A fungus produces the toxin Aflatoxin B1. It is a naturally occurring microbial carcinogenic agent that develops on the surface of grains, peanut butter, and various nuts. Hepatitis B and human papillomaviruses can also cause cancer in people who are infected with them.
There are also other carcinogenic compounds besides viruses, fungi, and radiations. Carcinogenic properties are also seen in compounds like benzene and polynuclear hydrocarbons with more than two benzene rings fused together. When organic materials such as tobacco, coal, and petroleum are incompletely burned, polynuclear hydrocarbons are produced. These compounds make their way into the human body, where they are subjected to biochemical reactions. This kills DNA cells, resulting in cancer, which can lead to death.
Toxicity is the degree to which a chemical can harm a person's body's human cells. We've seen how carcinogenicity works and the compounds that are linked to it. These compounds are extremely harmful by nature, and their use should be avoided if you want to keep your body safe.
4.6.1 The disease: present status
Malaria is one of the most visually striking and geographically common re-emerging infectious diseases. The disease affects 40 percent of the world's population, or more than two billion people, in 100 countries, with 300–500 million cases and 2–3 million deaths each year, mainly among children. Plasmodium falciparum, the most dangerous of the four protozoan species that commonly infect humans, causes the most serious clinical cases of malaria, including coma (cerebral malaria), profound anaemia, renal failure, and death. While the disease has had an effect on the evolution and history of the human race, causing widespread human suffering, parasite and host resistance is the disease's main challenge today. This problem emerged as a result of a five-decade-old drive to eradicate the disease by using anti-plasmodial chloroquine (CQ) and anopheline vector insecticide DDT. Inadvertently, the campaign paved the way for parasite resistance, which is spreading and rendering the only affordable anti-malarial drugs useless, as well as vector resistance to insecticides. Resistance to CQ is common in Southeast Asia, South America, and Sub-Saharan Africa, while resistance to fansidar (FN) is common in South America and Southeast Asia. Multi drug resistance (mdr) of Plasmodium falciparum (to CQ, mefloquine, FN, and to a lesser extent, quinine and guanidine) is a major problem in Southeast Asia and has also spread to South America, while CQ resistance in Plasmodium vivax has recently emerged. The long-awaited vaccine, despite making some strides, is still years away from clinical use. Similarly, developing a vector control strategy focused on genetic modification of vector competence in wild vector populations is still a long way off. Due to the limited progress made with conventional methods, the only practical alternative is the production of novel drugs (aimed at particular parasite targets) and novel treatment regimens based on drug combinations (aimed at reducing resistance induction). The fact that resistance is frequently just a few steps behind drug application has created a serious quandary on how any new drug, no matter how effective, can be used.
4.6.2 Drug therapy
The current arsenal of anti-malarial drugs includes a wide range of agents, but the most powerful seem to be those that influence processes related to haemoglobin (Hb) digestion in the parasite's food vacuole (Fig. 1). This is a one-of-a-kind and crucial process for parasite growth, and the primary purpose of Hb digestion appears to be to provide ‘room' for parasites that are rapidly growing and dividing. Parasites may be able to use some of the catabolites as nutrients while neutralising the effects of the ones that are clearly harmful (Fig).
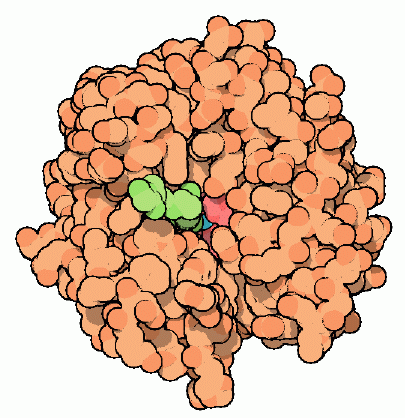
Intra-erythrocytic parasites' Hb digestion properties. Within the Hb-rich host cell, the parasite (P) is enclosed. Hb, along with other host components, is taken up by the parasite and transmitted to the parasite food vacuole through endocytosis (fv). The host-ingested Hb is degraded sequentially by the indicated Asp and Cys proteases in an acidic environment provided by an H+-ATPase in the fv. Part of the heme released is polymerized into hemozoin (Hz), while the rest can exit the fv and generate free Fe. Membrane transporters and/or simple diffusion systems appear to transport products of Hb digestion, such as amino acids (aa) and unpolymerized heme, into the parasite cytosol.
The uniqueness of that process is due to properties associated with the food vacuole, such as compartmentalised acidity and the expression of particular proteases for Hb degradation as well as mechanisms for neutralising the released toxic heme. Both heme polymerization into hemozoin and glutathione-mediated heme degradation have been proposed as mechanisms for the latter, with the first being linked to the food vacuole and the second to the parasite cytosol. The chemical or biochemical basis of those pathways, on the other hand, has yet to be determined. Nonetheless, it is clear that drugs that interfere (directly or indirectly) with either of the above processes of Hb digestion (e.g., CQ, anti-proteases, etc.) and/or heme neutralisation can jeopardise parasite survival (e.g., CQ, pro-oxidants). Furthermore, both heme, a product of Hb degradation, and iron, a putative product of GSH-mediated heme destabilisation, have been implicated in the catalysed development of toxic radicals by anti-oxidant drugs like artemisinin (Fig. 2). Due to the lack of mechanisms for iron absorption in mature red cells, rapidly growing parasites appear to rely on Hb degradation for a steady supply of the metal, making them susceptible to chemotherapeutic attack by iron chelators.
On parasite components, the site of action of the main anti-malarial drugs (AMDs). Long arrows (right) show the various drugs acting on different aspects of Hb degradation within the parasites, and their putative parasite targets are shown on the left side of the scheme. PfMDR1 and CG2 are putative drug transporters, and their presumed transport activities have been linked to parasite mdr products.
4.6.3 Iron, infection and metabolic immunity
The theory behind metabolic immunity is that depriving a host of an essential nutrient from an invading organism can function as a host defence mechanism as well as a therapeutic strategy (if tolerated by the host). Early research with iron-deficient Somali nomads revealed a 5-fold rise in infection episodes (tuberculosis, malaria, and brucellosis) in the iron-treated group versus the placebo group. Patients who were given a better diet when they entered hospitals had similar results. While it is widely recognised that iron overload can cause the host to become vulnerable to infection, only a few cases of the reverse have been demonstrated in the natural setting of iron deficiency. Malaria, pneumocystis, and a few fungal diseases are among them.
4.6.4 Iron and malaria
Malaria parasites, like all living organisms, require iron for essential cell functions and must manage their cellular contents in a highly regulated manner. The relationship between the host's iron status and microbial infection has long been recognised. Microorganisms such as bacteria and fungi depend on extracellular iron (III) for growth and reproduction regardless of the environment. They get non-penetrating iron (III) by secreting siderophores, which are low-molecular-mass, water-soluble structures that scavenge extracellular iron (III) with high affinity, bind to specific surface receptors as iron complexes, and help iron internalisation through a multi-step mediated process. While no conclusive clinical success with bacterial infections has been identified, interference with those processes by bacterial receptor antagonists or iron deprivation at the host level has shown some differential effects on bacterial development. The general theory is that normal human tissue can tolerate temporary iron deprivation better than rapidly growing tumours, so iron chelation may be useful for cancer chemotherapy. Other applications have been proposed for treating conditions like cardiac ischemia reperfusion and Adriamycin cardiotoxicity, which are both linked to iron-mediated cell damage. Above all, iron chelation therapy has proved to be highly effective and is still the most common treatment for iron overload.
Parasites have a unique case of iron metabolism during their asexual stage of development. They have no apparent means of mobilising bioavailable iron from the medium through the host, despite living in a sea of Hb and carrying within the remnants of its degradation products (Fig. 1). They differ from most mammalian cells in that they are exposed to body fluids and obtain metal from circulating iron carriers. The uniqueness of parasite iron handling is manifested in their resistance to drug-induced iron deprivation. What is the basis for iron (III) chelators' selective cytotoxicity, and what is the potential of iron chelation therapy in the treatment of malaria?
4.6.5 Iron chelators as anti-malarial
Desferrioxamine (DFO)
Due to the malaria parasite's high replication rate and presumed high iron utilization, attempts have been made to cause selective "iron starvation" of parasites using the hydroxamate-based DFO, a natural siderophore and an iron (III) chelator that is currently used in clinical trials for iron overload diseases. The method seemed promising at first, as the agent inhibited P. falciparum growth in vitro and suppressed malaria proliferation in mice, monkeys, and, more recently, humans. Inasmuch as DFO, like all other hydroxamates, had little effect on transferrin iron but demonstrated unique access into the inner compartments of infected cells, the source of iron affected by this highly specific iron chelator was hypothesised to be intracellular, probably intraparasitic. The drug's developmental stage of action was determined to be trophozoite/schizont, with the iron-dependent enzyme ribonucleotide reductase as the main putative goal (RNRase). DFO inhibits enzyme activity most likely by scavenging iron (III and II) from the iron reservoir with which the enzyme is in dynamic equilibrium. DFO may also function on parasites by destabilising the iron-containing hemozoin, resulting in the release of toxic heme-derived material, according to one theory. Due to reduced membrane permeation across biological membranes, DFO had a significant disadvantage in terms of speed of action in biological systems.
New chelator design
The anti-malarial efficacy of the relatively safe DFO chelators could be improved by making hydroxamate-based drugs more permeant to infected cells. To achieve that goal, two methods were used, both of which were based on modular chemical approaches that allowed for systemic changes to improve drug performance. One is exemplified by a class I of synthetic hydrophobic siderophores, namely reversed siderophores (RSFs), which have an iron binding cavity biomimetic to that of ferrichrome, and the other, class II, is exemplified by N-terminal derivatives of DFO, which retain the parent compound's iron (III) binding capability. The lipophilic character of the amino acid chains emerging from the basic polyhydroxamate structure regulated the
hydrophobic/hydrophilic balance of the RSFs, while changing the size of the connecting arms modulated the molecular dimensions. The probes' permeation through biological membranes was demonstrably aided by these chemical properties. The class II was made up of DFO N-terminal derivatives with increased membrane perm selectivity thanks to the addition of hydrophobic moieties. Bidentate hydroxypyridones (HPO), which form 3:1 complex with iron but are orally active for treating iron overload in animal models and clinical trials, were classified as class III agents. Orally active agents based on various hydrazone adducts of aromatic and heterocyclic aldehydes that form 2:1 iron complex was included in class IV (labelled XIH for X-substituted isonicotinoyl hydrazones). Natural dihydrochalcones present in plants were the last (class V). All of the identified agents were tested first in vitro on P. falciparum-infected erythrocyte cultures, then in animal models of malaria, and finally in clinical trials. Table 1 shows the anti-malarial properties of the five groups of iron chelators.
Table 1
Anti-malarial activity of various classes of iron chelators
Chelator | Class | IC50 in vitro (µM) | Rodents |
DFO | I | 10–30 | sc, ip, iv |
X-DFO | I | 3.0–30 | NA |
RSF-tripodal | II | 0.3–20 | sc |
HPO | III | 10–50 | iv |
XIH | IV | 0.2–30 | sc, ip |
DiOH-coumarin | V | 0.8–20 | sc, ip |
The growth test was based on nucleic acid synthesis, which occurs most frequently at the trophozoite-schizont stage, and the IC50 values of the indicated classes of drugs were for a 24–48-h drug exposure in a mixed in vitro culture containing primarily trophozoites (>70 percent), and the IC50 values of the indicated classes of drugs were for a 24–48-h drug exposure in a mixed in vitro culture containing primarily trophozo RSF-tripodal refers to hydroxamate-based RSFs, HPO to hydroxypyridinons, XIH to aryl-aldehyde-isonicotinoyl hydrazone adducts, and diOH-coumarin to daphnetin, a bioflavonoid chelating agent. The indicated drugs were administered intravenously (iv), intraperitoneally (ip), and subcutaneously (sc) to rodent models of malaria (NA=not applied) and were successful in reducing parasite burden or parasitaemia.
The anti-malarial potency of at least class I and II chelators did not differ among P. falciparum strains with a broad range of susceptibility to CQ and other drugs. The lipophilicity of the drug was the most important factor in the anti-malarial activity profiles of three groups of iron chelators (Fig. 3). The variations in inhibitory potency among the chelator families cannot be explained solely by their capacity for bulk iron extraction from cells, since all of the agents used are highly potent iron binders (pKa>25). Rather, they suggest that more subtle properties, such as differential accessibility to hydrophobic pockets of important parasite iron-containing enzymes, are involved in their mode of operation (e.g., RNRase, cyclooxygenases).
The effect of drug lipophilicity on the structure-activity relationships of three groups of iron chelators as anti-malarials. The drugs were tested in vitro on P. falciparum development, and the IC50 values were calculated after a 48-hour exposure to the drugs, starting at the mid-ring level. The abscissa denotes the drugs' related chemical properties, which are calculated by the product of relative iron-drug binding affinity and drug partition coefficient (modified from with additional unpublished data on HPOs obtained by Haupt, Hider and Cabantchik). DFO derivatives (MA=methylanthranilic and NBD=nitrobenzyldiazole), different hydroxypyridinons (HPO), and substituted RSFs with different amino acids and connecting arms (m1 and m2) and/or ester groups were the three families of chelators studied (OEt, for ethyl).
Specificity of action
To the extent that the above iron (III) chelators' efficacy on malaria parasites is based on their ability to cause a deficiency in bioavailable cell iron, this property is likely to occur to some degree in other cells of the host organism. As a result, the rationale for treating malaria with drug-mediated iron deprivation must presume that the host and parasite have different levels of drug tolerance. In practise, chelators must also meet the requirement of parasite cytotoxicity specificity versus host organism in order to be effective curative agents. Most mammalian cells tested for drug-induced growth arrest were found to be in this state. Despite the fact that almost all of the chelators used stopped the mammalian cells from growing, they resumed normal growth after the medication was removed and their serum iron pools were replenished (i.e., cytostatic action).
Regardless of whether exogenous iron was re-supplied following drug treatment or not, parasites demonstrated a limited ability to recover after being exposed to iron chelators. Both the growth inhibitory properties and the ability to recover from iron chelation were highly dependent on the parasite's developmental stage and the type of drug treatment. In terms of cell results, these properties are referred to as cytostatic or cytotoxic, and reversible or irreversible in terms of drug mode of inhibition.
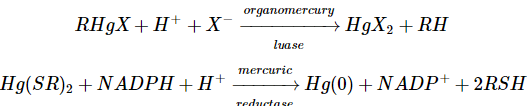
Chelators' cytostatic and cytotoxic (persistent) effects in relation to parasite developmental stage and drug intake windows. Parasitized cells (2 percent haematocrit, 10% parasitaemia) were exposed to 100-M concentrations of the specified chelators (DFO or SIH) for the time periods indicated, and nucleic acid synthesis ([3H] hypoxanthine incorporation) was measured during the last 3 hours of drug exposure (for continuous drug exposure) or 3 hours after drug removal (discontinuous exposure). The percent inhibition values were calculated using the formula 100-[3H] percent integration in comparison to the parallel control (no drug treatment).
The anti-malarial effect of DFO, which is poorly permeant to rings, was largely irreversible and only manifested in the advanced developmental stages. The discovery that a significant fraction of the drug taken up by cells was retained in trophozoites and schizonts even after drug removal was linked to the sustained biological impact of the relatively slow permeating DFO. The results of salicylaldehyde isonicotinouyl hydrazone (SIH) (Fig. 4) or RSFs, on the other hand, were mainly irreversible in rings but more reversible in trophozoites. The findings of the previous studies were combined into a working model (Fig. 5).
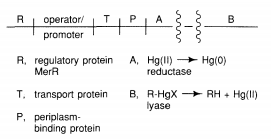
In malaria parasites, a model of iron chelator action is shown. Both stage permeant SIH and RSF, as well as the trophozoite-schizont permeant DFO, affect malaria parasite iron utilisation (shown in the box). The chelators' permeation is represented by straight upward pointing arrows. The active phase of iron mobilization (from Hb degraded in the food vacuole) is indicated as slant arrows and the stage of RNRase-dependent DNA synthesis as a box in the trophozoite stage. The lower boxes indicate whether the parasite is susceptible to the drug in a reversible (empty box) or irreversible (filled box) mode at the time of growth. The minimum exposure time needed to produce >90 percent irreversible inhibition by 100 M drug in one cycle of growth is represented by the empty fraction of the filled package.
The most active process of iron mobilisation and metabolic integration by parasites is associated with trophozoites and early schizonts, according to the model. Parasites obtain metabolic iron mainly from Hb degradation, which is most active during these parasite stages. The ring stage's demand for iron, on the other hand, can only be met by metal mobilisation from the host's labile iron pool, which is both small and seemingly irreplaceable if depleted. Iron chelators' anti-malarial effectiveness is based on their ability to cause intracellular iron depletion and irreversible damage to parasites by accessing (a) ring types, even for a fraction of the developmental stage, and/or (b) trophozoites, for the entire active iron process, to counteract the iron released by Hb degradation. Also, for a membrane permeant chelator to be an effective anti-malarial, the minimum drug exposure period in the blood is 6–8 h even at relatively high drug doses, according to the model. This has implications for their potential therapeutic use of anti-malarials, for example. Only relatively long drug exposures will increase the anti-malarial efficacy of hydrophilic agents like DFO, which have limited access to rings and trophozoites (i.e., continuous drug administration at relatively high doses). The partial cytotoxic existence of permeant agents like SIH or RSF's effects on trophozoite-schizonts can only be resolved by using them as permeant pro-drugs that produce less permeant derivatives intraparasitically. However, it's possible that the combined action of the two groups of drugs will make the parasite vulnerable at all stages of its growth, boosting the drugs' anti-malarial ability beyond their theoretical additive effects. The latter was deduced from the fact that SIH and RSF's quick (and even partially reversible) cytostatic action on trophozoites and schizonts would potentiate the irreversible DFO effect by allowing more DFO to be taken up into the advanced stages of parasite growth, preventing the parasite growth cycle from being completed. In vitro experiments with P. falciparum cultures (Fig. 6) and animal model research also confirmed these predictions experimentally (P. vinckei petteri in mice). The latter has also given indirect evidence for chelators' anti-malarial effect by relieving parasite-impaired macrophage phagocytoses.
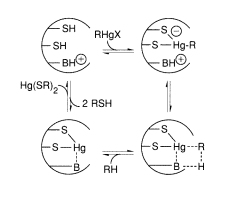
SIH and DFO have individual and combined (synergistic) effects on P. falciparum development. A: Parasite cultures (mid-term rings) were exposed for 24 hours to either drug (SIH 2–100 M, open squares; DFO at constant 10 M, filled squares), or both drugs (filled squares), and nucleic acid synthesis was monitored for another 24 hours in the presence of drugs. 100 percent growth=0 percent inhibition was used to measure the percent inhibition using the value of the control (untreated parasitized cells). The lines drawn over the experimental points show that neither the 'additive' (broken line) nor the 'independent' (dotted line) modes of combined drug-drug action apply to SIH+DFO, leaving drug synergy as the most viable mode of combined drug action. B: As in A, but SIH was 5 M and DFO was 10 M, and nucleic acid synthesis inhibition was tracked over time. The synergistic mode of action of the combined drugs was verified.
4.7.1 Haemoglobin:
What is haemoglobin and how does it work?
Haemoglobin is a globular protein found in red blood cells (RBCs) that transfers oxygen across the body via the bloodstream. The heme prosthetic group is attached to each subunit of this tetrameric protein. It is a respiratory pigment that aids in the transport of oxygen from the lungs to various parts of the body as oxyhaemoglobin. Carbon dioxide is also transferred back into haemoglobin in the form of carbaminohaemoglobin.
Myoglobin in muscles, haemocyanin in arthropods and mollusks, leghaemoglobin in legumes, and other oxygen-binding proteins are examples.
Haemoglobin is a protein that carries oxygen in the body. HBA1, HBA2, and HBB genes code for a present in humans. The amino acid sequence in Hb polypeptide chains varies between organisms.
Hb heme is made in the mitochondria and cytoplasm of immature RBCs. Ribosomes produce the globin protein in the cytoplasm. The residual rRNA in mature mammalian RBCs continues to synthesise Hb until the reticulocytes reach the vasculature, even after the nucleus has been lost.
The level of haemoglobin in the blood is measured in g/dL. The amount in a healthy person varies from 12 to 20 g/dL. In general, males have a higher Hb level than females. Males have a normal level of 13.5 to 17.5 g/dL, while females have a normal level of 12 to 15.5 g/dL.
Let's take a closer look at haemoglobin's structure and work.
Structure of Haemoglobin
In 1959, Max Perutz discovered the molecular structure of haemoglobin. The protein haemoglobin is tetrameric. In adults, the main form of haemoglobin is made up of two polypeptide chains, each with two subunits. Each polypeptide chain has a heme prosthetic group attached to it.
subunit – It is made up of a 141-amino-acid-residue alpha polypeptide chain.
subunit – It is made up of a 146-amino-acid-residue beta polypeptide chain.
Heme group – Each polypeptide chain has an iron-containing prosthetic group attached to it. The porphyrin ring contains iron in the middle.
There is a close interaction between and subunits in the quaternary system. Haemoglobin partially dissociates after a mild urea injection, but dimers remain intact. Hydrophobic interactions, hydrogen bonding, and a few ion pairs or salt bridges bind the subunits together.
There are two alpha and two gamma chains in children, which are gradually replaced by beta chains.
There are two types of haemoglobin conformations: R state and T state. Deoxyhaemoglobin is mainly present in T state, while oxygen has a stronger affinity for R state.
Function of Haemoglobin
Hb's primary role is to carry and transport oxygen to different tissues. Oxygen binding to Hb is a cooperative process. The transition between the low oxygen affinity T state (Tense) and the high oxygen affinity R state (Ren) is responsible for the binding and release of oxygen from Hb in the lungs and tissues, respectively (Relaxed).
Transport of oxygen
pH, 2,3 BPG, and BPG influence the affinity of oxygen for Hb (2,3-Bisphosphoglyceric acid). T-state is favoured in tissues with low pH, high BPG, and CO2, and oxygen is released, while R-state is favoured in the alveoli with high pH, low CO2, and BPG concentrations, resulting in oxygen binding to Hb.
The partial pressure of oxygen also influences oxygen binding. When pO2 is high in the lungs, oxygen binds with Hb, and when pO2 is low in the tissues, oxygen is released.
Each 100 millilitres of oxygenated blood carries 5 millilitres of oxygen to the tissues.
As the first oxygen molecule binds to the heme unit of one deoxyhaemoglobin subunit (T-state), conformational changes occur, resulting in an improvement in affinity, and the second molecule binds more quickly. When the fourth molecule is already in the R state, it binds to it. The sigmoid curve of oxygen binding to Hb can be seen.
Allosteric binding is a form of binding in which binding at one site affects the affinities of the other binding sites.
The pulse oximeter is a device that tests the amount of oxygen in the blood. It's used to figure out whether you're suffering from hypoxia. It is based on the fact that the absorption spectra of oxyhaemoglobin and deoxyhaemoglobin are different. This is a crucial method that doctors use to monitor COVID-19 patients' oxygen saturation levels, as well as those that are at risk.
Transport of Carbon dioxide
Around 20-25 percent of CO2 is transported as carbaminohaemoglobin attached to haemoglobin. Carbon dioxide binding is favoured in tissues where pCO2 is higher and pO2 is lower, while carbaminohaemoglobin dissociation occurs in the alveoli due to high pO2 and low pCO2. The remaining CO2 is transported as bicarbonate, which is aided by an enzyme known as carbonic anhydrase.
4 mL of CO2 is carried to the alveoli for every 100 mL of deoxygenated blood.
Nitric oxide bound to the globin protein is also transported by haemoglobin. It binds to the thiol groups in the chains of globin.
The carboxyhaemoglobin complex is formed when carbon monoxide binds to haemoglobin. Carbon monoxide has a 250-fold higher affinity for haemoglobin than oxygen. As a result, even the tiniest amount of CO will affect oxygen binding. As a result, breathing CO-rich air can cause headaches, nausea, and even unconsciousness. In heavy smokers, it can block 20% of active oxygen binding sites.
● Diseases related to Haemoglobin
Haemoglobin deficiency can be caused by a variety of factors. The blood's oxygen-carrying ability is reduced as a result of haemoglobin deficiency. It may be caused by a lack of nutrients, cancer, kidney failure, or genetic defects.
Haemoglobin levels that are higher than average is linked to a variety of heart and pulmonary disorders.
Sickle cell anaemia is caused by a haemoglobin gene mutation. The globin chain has a single nucleotide or point mutation. Glutamic acid is replaced by valine at the 6th position after ‘GAG' is converted into ‘GTG.'
Thalassemia is caused by a decrease in haemoglobin production. -thalassemia and -thalassemia are the two forms of thalassemia. It is often caused by faulty genes, and the magnitude is determined by the number of genes that are absent or defective.
The haemoglobin level is a popular diagnostic tool. The HbA1c level, or glycosylated Hb or Hb linked to sugar, is a marker for a diabetic patient's average glucose level in the blood.
To summarise, haemoglobin is a necessary pigment for the transport of oxygen and the performance of normal bodily functions.
4.7.2 Myoglobin:
Myoglobin (abbreviated Mb or MB) is an iron- and oxygen-binding protein present in the skeletal muscle tissue of vertebrates in general and almost all mammals in particular. Haemoglobin and myoglobin are unrelated. Myoglobin has a higher propensity for oxygen than haemoglobin and does not have mutual binding with oxygen like haemoglobin. It is, however, an oxygen-binding protein found in red blood cells. Myoglobin is only present in the bloodstream following a muscle injury in humans.
Muscle cells with high myoglobin concentrations will hold their breath for longer periods of time. Myoglobin is especially abundant in the muscles of diving mammals such as whales and seals. Myoglobin is present in Type I, Type II A, and Type II B muscle, but it is not found in smooth muscle, according to most texts.
X-ray crystallography showed the three-dimensional structure of myoglobin for the first time. John Kendrew and colleagues wrote on this achievement in 1958. Kendrew and Max Perutz shared the Nobel Prize in Chemistry in 1962 for this discovery. Despite being one of biology's most studied proteins, its physiological role is still unknown: mice genetically engineered to lack myoglobin may be viable and fertile, but they exhibit several cellular and physiological adaptations to compensate for the loss. It is hypothesised that myoglobin function is related to increased oxygen transport to muscle and oxygen storage, as well as acting as a scavenger of reactive oxygen species, after witnessing these changes in myoglobin-depleted mice. Myoglobin is encoded by the MB gene in humans.
Myoglobin can be oxymyoglobin (MbO2), carboxymyoglobin (MbCO), or metmyoglobin (met-Mb), just as haemoglobin can be oxyhaemoglobin (HbO2), carboxyhaemoglobin (HbCO), or methemoglobin (met-Mb) (met-Hb).
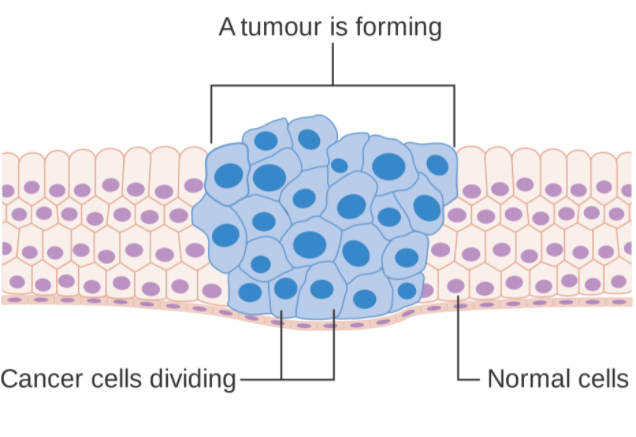
Myoglobin
4.7.3 Differences from haemoglobin:
Myoglobin is a cytoplasmic protein that binds oxygen to a heme group, similar to haemoglobin. Haemoglobin has four globulin groups, although it only has one. Mb has a higher affinity for oxygen than haemoglobin, despite the fact that its heme group is similar to that of Hb. This distinction is due to its different function: while haemoglobin transports oxygen, myoglobin stores it.
Key takeaway:
References:
1. J. M. Chilton and J. J. Fardy, J. Inorg. Nucl. Chem., Vol. 31, 1171-1177, (1969).
2. N. A. Talvitie, Anal. Chem., Vol. 43, p 1827, (1971).
3. N. Shinohara and N. Kohno, J. Nucl. Sci. & Techol., Vol. 34(4), 398-401, (1997).
5. M. Y. Suh, et al. KAERI/TR-2354/2003, (2003).
5. M. V. Ramaniah, et al., BARC-736, (1974).
6. F. Nelson et al., J. Chromatog. 14, 258-260 (1964).