Unit - 4
Pharmaceutical Compounds: Structure and Importance
4.1.1 Pharmaceutical meaning:
Pharmaceuticals are substances that are used to diagnose, cure, or prevent disease, as well as to restore, rectify, or modify organic functions.
Ancient Chinese, Hindu, and Mediterranean civilizations all have records of therapeutic plants and minerals. Galen and other ancient Greek physicians employed a wide range of medications in their work. Pharmaceutical practise began to expand fast in the 16th century AD, after Western medicine awoke from its long slumber during the Dark and Middle Ages. The first pharmacopoeia (catalogue of medications and their preparations) was published in 1546 in Germany, and the profession of pharmacy is thought to have originated in 1617 in London with the foundation of the Society of Apothecaries. Anaesthetics were among the first modern medications, with morphine being used in around 1804, ether in 1842, chloroform in 1847, and cocaine in 1860. Strychnine (1817), quinine (1820), and nicotine were among the other chemicals discovered in the nineteenth century (1828). In 1865, Joseph Lister was the first to utilise phenol (carbolic acid) to prevent infection.
Pharmaceuticals are often divided into three categories: chemical groups, pharmacological effects, and therapeutic uses. Quinine, nicotine, cocaine, atropine, and morphine are examples of alkaloids, which were the first pure medications created from natural sources (plants). Animal-derived drugs include glandular extracts containing hormones, such as insulin for diabetes treatment.
Other major medications made from natural sources include antibiotics, vaccines, human blood-plasma fractions, and steroid hormones. Vitamins, which were once derived from natural sources, are now frequently synthesised in laboratories.
4.1.2 Pharmaceuticals in Indian drinking water
Larsson and his colleagues found metoprolol, enoxacin, enrofloxacin, citalppram, norfloxacin, lomefloxacin, ciprofloxacin, losartan, cetirizine, ofloxacin, and ranitidine in the wastewater of a sewage treatment plant at Patancheru, Hyderabad.
Table lists the pharmaceutical chemicals found in ground and surface water at detectable concentrations. In another investigation, the average total concentration of pharmaceutical chemicals discovered in each sample was determined to be 24 ng/L. Antipyrine (analgesic) and sulfamethizole (antibiotic) were found in drinking water for the first time in the United States. Fick et al. Discovered drugs in samples taken from wells, lakes, and rivers in the vicinity of Hyderabad, India (Table 3). The authors discovered that all of the wells were contaminated with drugs such as Ciprofloxacin, enoxacin, cetrizine, terbinafine, and citalopram in concentrations greater than 1 mg/L, while higher concentrations of ciprofloxacin (6.5 mg/L), norfloxacin (0.52 mg/L), enoxacin (0.16 mg/L), and cetirizine (1.2 mg/L) drugs were investigated in two lakes
Table:
Pharmaceutical compounds with detected concentrations in different water bodies with their extraction methods.
Pharmaceutical compound | Concentration | Extraction method | Instrument used | Country | References | |
Ground water | ||||||
Acetaminophen | 1.89 mg/L | Solid phase extraction | HPLC-MS | USA | 42 | |
Caffeine | 0.29 mg/L | |||||
Carbamazepine | 0.42 mg/L | |||||
Codeine | 0.214 mg/L | |||||
P-xanthine | 0.12 mg/L | |||||
Sulfamethoxazole | 0.17 mg/L | |||||
Trimethoprim | 0.018 mg/L | |||||
Surface water | ||||||
Ibuprofen | 414 ng/L | Solid phase extraction using high performance extraction disks (SBD-XD) | HPLC with tandem MS | South Korea | 43 |
|
Carbamazepine | 595 ng/L |
| ||||
Atenolol | 690 ng/L |
| ||||
Clarithromycin | 443 ng/L |
| ||||
Mefenamic acid | 326 ng/L |
| ||||
Erythromycin | 137 ng/L |
| ||||
Fluconazole | 111 ng/L |
| ||||
Levofloxacin | 87.4 ng/L |
| ||||
Indomethacin | 33.5 ng/L |
| ||||
Propranolol | 40.1 ng/L |
| ||||
Ifenprodil | 35.4 ng/L |
| ||||
Finofibric acid | 3.20 mg/L | Oasis HLB solid phase extraction | Reverse Phase HPLC through diode array detector with C18 column | Douro River Estuary | 44 |
|
Carbamazepine | 0.60 mg/L |
| ||||
Diazepam | 1.60 mg/L |
| ||||
Fluoxetine | 32.00 mg/L |
| ||||
Propranolol | 0.80 mg/L |
| ||||
Sulfamethoxazole | 1.40 mg/L |
| ||||
Trimethoprim | 8.00 mg/L |
| ||||
HPLC-MS, high-performance liquid chromatography coupled with mass spectrometry; SBD
Table:
Detection of pharmaceuticals in fresh water samples in Hyderabad area, India.
Drug | Lakes(03 sampling Sites Of 02 lakes) | River (06 Sampling points) | Wells (06 wells) |
Norfloxacin | 60,000-520,000 | ND-4700 | ND-31 |
Ciprofloxacin | ND-6,500,000 | 10,000-2,500,000 | 44-14,000 |
Ofloxacin | ND-11,000 | 180-10,000 | ND-480 |
Enoxacin | 14,000-160,000 | ND-66,000 | ND-1900 |
Enrofloxacin | ND-25,000 | ND-30,000 | ND-67 |
Metoprolol | 7000-ND | ND-240 | ND-90 |
Cetirizine | 5000-1,200,000 | 5,400-530,000 | 550-28,000 |
Citalopram | 2000-8000 | ND-76,000 | ND-1400 |
4.1.3 Classification of Drugs
Classification of drugs can be done on the basis of certain criteria. Some of them are given below.
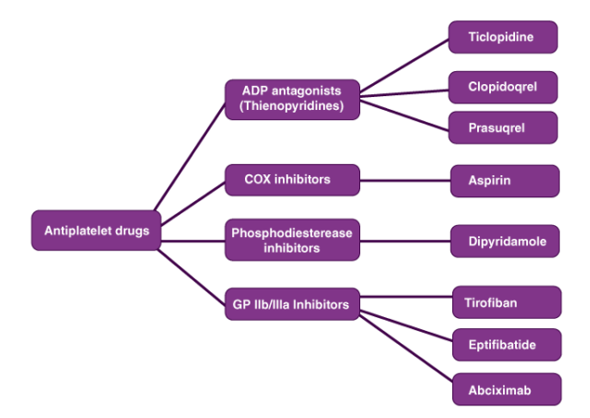
Classification of Drugs on the basis of the Pharmacological Effect:
- How a drug or medicine affects or influences the cells of an organism is referred to as the pharmacological effect. Different types of drugs have various pharmacological effects on an organism.
- For example, an analgesic reduces pain while an anti-inflammatory drug reduces the inflammation of the body. Thus, drugs can be classified based on the pharmacological effect.
Classification of Drugs on the basis of Drug Action:
• Varied medications have different ways of eliciting a reaction, which is referred to as drug action.
• Drug action is more defined in terms of how it causes a response. There are several drugs to treat hypertension, for example, but each type of drug has a different function.
• All hypertension medications lower blood pressure, although in different ways.
Classification of Drugs on the basis of Chemical Structure:
This is a frequent drug classification. Drugs with the same drug action and pharmacological effect usually have the same fundamental skeletal structure with minor branching variations. As a result, certain medications have greater potential than others. Sulphonamides, for example, all have the same skeleton structure.
Classification of Drugs on the basis of Molecular Targets:
Drugs target macromolecules inside the body to elicit a biological reaction, which are known as target molecules or drug targets. The target of drugs with the same mechanism of action will be the same. This method of medication classification is more useful during clinical trials.
4.1.4 Chemical Stability of Pharmaceutical Organic Compounds:
Penicillin
Hydrolytic enzymes like penicillin acylase, obtained from E. Coli, have been shown to be capable of catalysing the hydrolysis of natural penicillin to create 6-aminopenicillanic acid. In the semi-synthetic manufacture of antibiotics, this acid is a crucial step. The serine hydroxyl group is converted into an ester group, according to experimental findings and structural studies of the enzyme. At other words, the serine hydroxyl group in the active centre of the enzyme functions as a nucleophile, and the enzyme is regenerated by attacking the serine acyl intermediate or acyl enzyme with a nucleophile.
Penicillin is known to be sensitive to acidic environments, which catalyse its decomposition. The proximity of the secondary chain carbonyl group to that of the -lactam ring is a potential mechanism to explain such degradation. The amide bond is broken when the acid reacts with the nitrogen intra-cyclic atom. The breaking is facilitated by the extra cyclic nitrogen atom, which donates its non-bonded electrons to the secondary chain carbonyl group, which then donates its electrons to the -lactam ring, resulting in the formation of a new ring and the breaking of the -lactam ring, resulting in the complete destruction of penicillin. Penicillinase has also been discovered to be a -lactamase enzyme that degrades penicillin by initiating the hydrolysis process of the -lactam ring to produce penicillic acid.
In the presence of metallic ions such as zinc (II) and cadmium (II), four conventional penicillins, such as amoxicillin, ampicillin, penicillin G, and penicillin V, can be degraded in methanol. The Lewis acid activates the lactam ring carbonyl group, enhancing the electrophilic nature of the activated lactam ring carbonyl group's carbon atom, and methanol reacts as the nucleophile.
Carbapenem
Because of their chemical properties, carbapenems are -lactam antibiotics with a strong activity against bacteria and a high resistance to most -lactams. As a result, they fall under the category of antibiotics used first in the treatment of severe nosocomial infections. Carbapenem thienamycin, a natural substance produced from Streptomyces cattleya, was used to produce these antibiotics. The structure of carbapenem decomposes via Hofmann degradation or nucleophilic substitution process, according to experimental findings (SN2).
Atracurium
A medicine like atracurium is destroyed by several routes including the Hofmann elimination reaction. This later is a spontaneous degradation which occurs into the plasma and tissues at the human body’s normal temperature and pH. In this regard, the inhibition of neuromuscular transmission by atracurium is caused by the presence of quaternary nitrogen located at each of the congested ends, which are linked by an aliphatic chain. The structure of atracurium shows two ester groups and an asymmetric centre in each terminal end. The Hofmann elimination reaction commonly known as Hofmann degradation takes place thanks to the presence of two ester groups. Such a reaction was observed at the two ends of atracurium.
Duloxetine
Duloxetine is a solid chemical molecule that is just slightly water soluble. This medication is used to treat depression and anxiety disorders in general. It's a treatment for urine incontinence. The storage conditions, chemical properties, and contaminants all affect the medicine's stability. Under alkaline conditions, experimental observation revealed the breakdown of duloxetine to matching compounds such as naphthol. The electronic doublet of the duloxetine oxygen atom is a viable mechanism for explaining the disintegration products. In other words, the delocalization of the oxygen atom's electronic doublet results in the creation of two types of resonance. The action of the base on the ionic intermediate chemical aids in the dissolution of the medication, and the synthesis of naphthol is aided by the presence of a water molecule. However, it has been discovered that the breakdown product under acidic conditions is not naphthol.
4.2.1 Antipyretic Therapy:
Definitions
Fever is "a state of elevated core temperature, which is often, but not necessarily, part of the defensive response of multicellular organisms (hosts) to the invasion of live (microorganisms) or inanimate matter recognized as pathogenic or alien by the host." The febrile response, of which fever is but 1 component, is a complex physiologic reaction to disease involving a cytokine-mediated rise in core temperature, generation of acute-phase reactants, and activation of numerous physiologic, endocrinologic, and immunologic systems. The rise in core temperature during fever is to be distinguished from the unregulated rise that occurs during hyperthermia, in which pyrogenic cytokines are not directly involved and against which standard antipyretics are largely ineffective. Antipyretics block or reverse fever's cytokine-mediated rise in core temperature, but do not affect body temperature in the afebrile state. They are to be distinguished from hypothermia agents (cryogens), which are capable of lowering core temperature even in the absence of fever.
Antipyretic drugs
The release of pyrogenic cytokines by inflammatory cells in response to some exogenous pyrogen (e.g., infection), induction of cyclooxygenase (COX) 2 activation of the arachidonic acid cascade, and enhanced biosynthesis of prostaglandin E2 (PGE2) by hypothalamic vascular endothelial cells are all important components of the fever physiologic pathway. PGE2 raises the hypothalamic thermal set point (Figure) by acting on thermoregulatory neurons in the preoptic area of the anterior hypothalamus, causing peripheral and thermogenic mechanisms to increase core temperature. Antipyretics might theoretically disrupt the fever response at any point along this pathway.
The salicylates (e.g., sodium salicylate and acetylsalicylic acid), ibuprofen and other nonsteroidal anti-inflammatory medications (NSAIDs), and the para-aminophenol derivative acetaminophen are the most often used medications to decrease fever today. Until the 1970s, nothing was understood about the mechanisms underlying any of these drugs' antipyretic effect. Milton and Wendlandt demonstrated in 1970 that when E series prostaglandins are injected into the cerebral ventricles of cats and rabbits, they cause a rapid onset of fever and that PGE2 is released within the brain during fever. These findings, together with Vane's findings that aspirin and other antipyretic medicines decrease prostaglandin synthesis, suggest that antipyretics lower fever predominantly by reducing PGE2 generation in the brain. However, not all experimental evidence collected since Milton, Wendlandt, and Vane's early work has confirmed this notion. PGE2 injections into relevant brain areas of animals capable of developing a febrile response to endotoxin, for example, do not always result in fever. Furthermore, infusions of salicylate into the ventral septal area of experimental animals reduce the feverish reaction induced by intraventricular injection of PGE, implying that the mechanisms of action of at least some antipyretic medications may entail more than simply inhibiting PGE synthesis.
By inhibiting COX, acetaminophen, aspirin, and the other NSAIDs appear to limit the conversion of arachidonic acid to PGE2. The production of PGE2 at key sites in the hypothalamus is widely regarded as a crucial step in the activation of the physiologic cascade that raises core temperature during the febrile response. COX-1, a constitutive isoform, and COX-2, a mostly inducible isoform that is undetectable in most resting cells, are the two isoforms of cyclooxygenase. The former triggers the formation of prostacyclin, which is antithrombogenic and cytoprotective, while the latter is a key modulator of the inflammatory response. The anti-inflammatory action of NSAIDs is thought to be due to inhibition of COX-2, while inhibition of COX-1 is thought to cause undesired side effects such as stomach discomfort.
The two COX isoforms have a comparable structure and catalytic activity. Both have about 600 amino acids, with 63 percent of them being in the same order. Their active sites are at the very top of a long, narrow, hydrophobic channel. With two exceptions, the amino acids that make up the channel, as well as catalytic sites and adjacent residues, are identical in the two isoforms. In COX-2, valine replaces isoleucine at positions 434 and 523, as it does in COX-1. Many, but not all, of the differences in the reactivities of the two isoforms can be explained by these differences. Aspirin, for example, acetylates both isoforms' serine 530. This prevents arachidonic acid from reaching the catalytic site of COX-1, resulting in irreversible inhibition of the enzyme. Arachidonic acid access to the active site is maintained after aspirin acetylation of serine 530 due to COX-2's larger hydrophobic channel.
The relative potencies of acetaminophen and NSAIDs as COX inhibitors in the peripheral and central nervous systems differ. Acetaminophen, for example, inhibits central COX almost as well as aspirin and 10% as well as indomethacin, but only 5% as well as aspirin and 0.02 percent as well as indomethacin. Acetaminophen's poor anti-inflammatory action is most likely due to its modest efficacy on peripheral COX.
An antipyretic drug's duration of effect is determined by its concentration at the site of action as well as whether it inhibits COX reversibly or irreversibly. Aspirin's antipyretic effect lasts until a new enzyme is produced at the site of action since it inhibits COX irreversibly. Other NSAIDs are reversible COX inhibitors, and as such, their effects should be proportional to their concentration at the site of action. Many NSAIDs, however, are chiral compounds, meaning they exist in both S- and R-enantiomers (e.g., 2-arylpropionic acid derivatives, ibuprofen, and ketoprofen). By being converted to the S-enantiomer in vivo, the R-enantiomer, which is 100 to 500 times less potent against COX-2 than the S-enantiomer, serves as a drug depot. As a result, racemic combinations of the two enantiomers, which are the form in which many NSAIDs are sold, have longer durations of action than would be expected based on the S-pharmacokinetics enantiomers alone. There is a necessary delay between the release of endogenous pyrogens and pyrogen-induced rises in core temperature because the fever cascade (Figure) needs time to effect heat retention and production processes. The time an antipyretic medicine reaches its site of action and core temperature begins to fall is delayed for identical reasons. The ability of arachidonic acid metabolites, such as PGE2, to down-regulate production of at least some pyrogenic cytokines may also impact this antipyretic latency period. COX inhibitors generate a counterintuitive increase in pyrogenic cytokine translation by decreasing PGE2 synthesis.
Many clinical contexts, antipyretic dosages and formulations, and different metrics of clinical efficacy have all been used in studies of the relative potencies of the various classes of antipyretic medicines. As a result, there is no way to do a full meta-analysis of the amassed data set. Several research comparing ibuprofen and acetaminophen in children with fever are, however, enlightening. Overall, they show that ibuprofen, when taken orally, is a more effective antipyretic than acetaminophen. The variation in potency is minor, and the antipyretic effects of the two medications have a comparable time course, with both medications peaking 3 to 4 hours after oral administration.
There are few paediatric research on the relative activity of various NSAIDs. The two forms of nimesulide were nearly similar in studies comparing oral (5 mg/kg per day) and rectal (100-400 mg/d) nimesulide to oral placebo and rectal acetaminophen (200-800 mg/d). Furthermore, when administered in doses ranging from 1 to 4 suppositories per day, 100 mg of rectal nimesulide appeared to be at least as effective as 200-mg acetaminophen suppositories, depending on individual needs.
In adults, only a few studies have compared the antipyretic effects of NSAIDs. Ibuprofen (800 mg orally) is an effective antipyretic in endotoxin-challenged adult volunteers when administered soon before or concurrently with the endotoxin challenge, and is superior than acetaminophen in decreasing the temperature of patients with sepsis. In endotoxin-challenged adult volunteers, intramuscular ketorolac (30 mg) was found to be as efficacious as acetaminophen (650 mg orally) in reducing fever. Oral nimesulide (200 mg) and dipyrone (500 mg) were more efficient than oral aspirin (500 mg) in decreasing fever in a single-dose crossover experiment comprising individuals with diverse febrile illnesses. Finally, when comparing rectal nimesulide (200 mg) to acetaminophen (500 mg rectally) and diclofenac (100 mg rectally) to placebo in clinical studies, the three drugs showed equivalent antipyretic effectiveness.
Toxic effects are one of the most essential qualities that distinguishes antipyretic medications. Aspirin, for example, has a one-of-a-kind ability to cause Reye syndrome, a childhood illness defined by hepatic failure and encephalopathy caused by mitochondrial oxidative phosphorylation inhibition. NSAIDs have a slew of severe side effects (Table 1), the most serious of which, renal failure and gastrointestinal bleeding, are caused by their ability to suppress COX. Nonselective COX inhibitors are particularly susceptible to such side effects. For example, people who take piroxicam, a medication with a high affinity for COX-1, are 11 times more likely to have an adverse gastrointestinal event than those who do not take NSAIDs. When compared to nonusers of NSAIDs, those who use naproxen, a medicine with a stronger affinity for COX-2, have just a 3 times higher risk of significant gastrointestinal adverse effects. Other characteristics that appear to raise the likelihood of gastrointestinal toxic effects in NSAID users include age over 60, a history of gastrointestinal disease, concurrent corticosteroid medication, and NSAID use for a long time. Toxic effects are most common during the first month of treatment. The results of a longitudinal endoscopic study of people who had been taking aspirin for a long period imply that the gastric mucosa's tolerance to the harmful effects of NSAIDs increases over time. As a result, the risk of significant problems associated with infrequent use of nonselective COX inhibitors may be underestimated by rates of adverse events associated with chronic consumption of such medicines. Volunteers who took low-dose aspirin (650 mg twice daily) healed aspirin-induced mucosal ulcers significantly faster than those who took high-dose aspirin (median time to healing, 1 week) (650 mg 4 times daily; median time to healing, 5 weeks). 66
Lesko and Mitchell randomised nearly 84,000 children (aged 8 months to 10 years) to oral ibuprofen (5 mg/kg or 10 mg/kg) or acetaminophen (12 mg/kg) every 4 to 6 hours in a comprehensive survey studying antipyretic medication hazardous effects, afterwards questioning parents about adverse medical occurrences. The average treatment time for their individuals was three days, during which they received six to ten doses of antipyretic medicines. During the trial, about 1% of participants in each group were hospitalised, the majority for infectious disease treatment. Four youngsters, on the other hand, were admitted to the hospital due to gastrointestinal haemorrhage. Ibuprofen had been given to all of them in two doses. In those using ibuprofen, the risk of hospitalisation for acute gastrointestinal bleeding was 7.2 per 100,000. Although no child in the acetaminophen group had to be hospitalised due to acute gastrointestinal bleeding, the hospitalisation rates in the two treatment groups were not substantially different. Among the 55,785 children who received ibuprofen, there were no cases of Reye syndrome, allergy, or acute renal failure.
Because acetaminophen has limited effect on peripheral COX, it has little effect on the stomach or kidneys. While glucuronidation and sulfation are the primary routes of acetaminophen metabolism, it is also metabolised to a lesser amount via the p450 2E1 pathway, which produces N-acetyl-p-benzoquinoneimine, a highly electrophilic metabolite (NAPQ1). NAPQ1 accumulates and binds covalently to cell proteins and DNA after the primary routes are exhausted. Acute hepatotoxicity occurs when such binding is substantial and affects hepatocytes. NAPQ1 is detoxified by conjugation to glutathione in normal circumstances. The risk of acetaminophen-induced hepatotoxicity rises dramatically when glutathione stores are decreased, such as during chronic ethanol misuse or famine.
While acute liver failure in the context of an acetaminophen-assisted suicide attempt is widely known, the risk of hepatic injury from acetaminophen taken in dosages within or slightly beyond the therapeutic range has just recently come to light (4 g in 24 hours). In a recent study of 71 cases of acetaminophen-induced hepatotoxicity, it was discovered that 30 percent of the cases were caused by unintentional overdoses in patients who were taking the medicine for pain relief. Too frequent dose, simultaneous intake of numerous acetaminophen-containing substances, and ingestion of cough and cold treatments not identified as having acetaminophen were all reasons for excessive dose.
4.2.2 Aspirin Structure:
Aspirin provides a number of advantages, ranging from pain relief to lowering the chance of catastrophic illnesses including heart attacks and strokes. Aspirin is a widely used medication. It comes in a variety of forms, including capsules, water-soluble pills, powders, and oral gels.
Aspirin (acetylsalicylic acid) is an anti-inflammatory and analgesic medication used to treat cardiovascular disease. The observation that thrombocytopenia-inducing drugs reduced metastases led to the investigation of aspirin as an anticancer treatment, with aspirin significantly reducing fibrosarcoma metastasis in animal models.
Aspirin's structure is shown below.
4.2.3 Therapeutic Uses of Aspirin:
Aspirin (acetylsalicylic acid) is a prescription drug used to relieve pain and inflammation. It's a type of non-steroidal anti-inflammatory medication.
It's also used to prevent blood clots, heart attacks, and strokes, as well as bowel cancer. While some studies have indicated that aspirin helps reduce the incidence of heart attacks and cancers of the intestines, stomach, and oesophagus, doctors are advised to use caution when taking aspirin as a preventive therapy because it might cause gastrointestinal bleeding and damage.
People with renal failure, liver disease, or haemophilia should consult a doctor before using aspirin. Some people use aspirin to get euphoric or as a form of self-harm by taking more than the recommended amount.
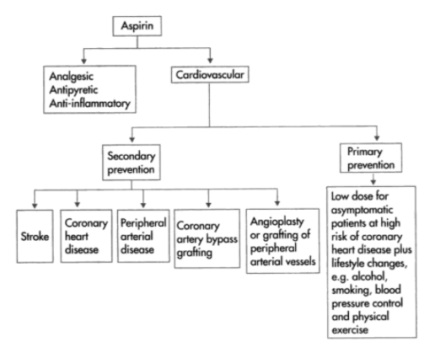
Aspirin increases the risk of bleeding in the liver, small intestine, and brain. The lining of your intestine and gut is generally protected from the acid in your stomach by a membrane. If aspirin is taken in high doses and for a long time, it will eventually harm this layer. The bleeding will be exacerbated by this damage. The use of aspirin to prevent blood clots will also hinder the normal mending of broken blood vessels and increase the risk of bleeding in the brain.
Key takeaways:
Pharmacologic Agents are a type of drug that can be used to treat a variety of on the basis of their modes of action, antipyretic medicines can be divided into three types. Corticosteroids, aspirin and other NSAIDs, and acetaminophen are among them
The antipyretic drug's main benefit is to make children more comfortable while also relieving parents' concern. Seizures that are febrile are normally harmless and do not result in brain damage. It is difficult to prevent and may not be possible. Antipyretics have no effect in preventing FS.
Most antipyretics currently appear to function by inhibiting the enzyme cyclooxygenase and lowering PGE (2) levels in the hypothalamus.
Paracetamol (acetaminophen; N-acetyl-p-aminophenol; APAP) is an analgesic and fever-reducing medicine:
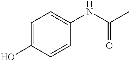
Many over-the-counter drugs, such as Tylenol and Panadol, contain it as an active component. It's also sold as a prescription drug in combination with narcotic pain relievers to treat more severe pain. It is the most widely prescribed pain and fever drug in the United States. It is listed as an essential medicine by the World Health Organization.
Acetaminophen is a coal tar derivative that works by interfering with the manufacture of prostaglandins and other molecules required for the transmission of pain impulses. It was first introduced in the early 1900s. Although it has a comparable effect to aspirin, it lacks the anti-inflammatory and blood-thinning properties of aspirin, is less stomach irritating, and can be taken by persons who are allergic to aspirin. Heavy use has been related to a higher risk of liver failure, particularly in alcoholics.
The original process begins with phenol, which is nitrated with sodium nitrate to produce an ortho- and para-nitrophenol combination. Distillation is then used to separate them. The para-nitro nitrophenol's group is subsequently converted to an amine, yielding para-aminophenol. After that, acetic anhydride is used to acetylate the amine:
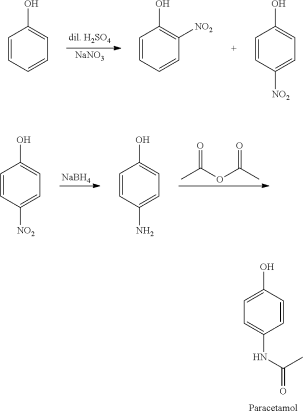
Direct acylation of phenol with acetic anhydride is an alternative industrial synthesis. The resultant ketone is subsequently transformed to a ketoxime using hydroxylamine, and the required amide product is obtained by an acid-catalyzed Beckmann rearrangement:
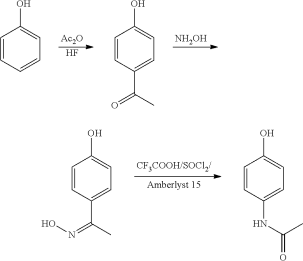
The initial reactant, phenol, is generated from fossil fuels in both ways. A long-felt and unmet need for a technology to produce paracetamol from a sustainable feedstock still exists.
4.3.1 Synthesis and crystallization:
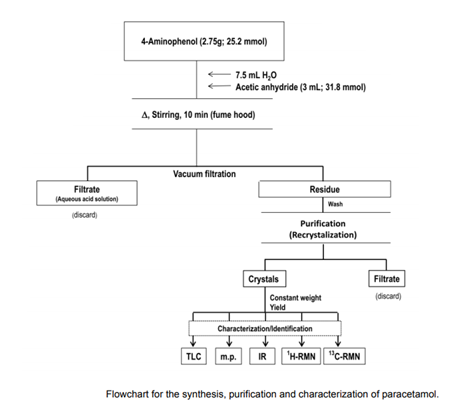
As a pre-lab exercise, students should create a table listing the physical parameters of 4-aminophenol, acetic anhydride (etanoic anhydride), and paracetamol (molecular formula, molecular mass, purity grade of the reagents, m.p., b.p., solubility, and theoretical and utilised mass in grammes and moles). A footnote providing the matching bibliography sources is required for this table. In addition, the instructor can teach students to create a chart similar to Figure,
The reaction is carried out in an Erlenmeyer flask, although it can also be carried out in a round-bottom flask with a reflux condenser if possible. If not, and for safety considerations, students must first secure the Erlenmeyer to a universal support with a clamp holder or hold it with a wood clamp. In this last case, the reaction can be carried out without the use of magnetic stirring. Water baths should be offered near boiling to shorten class time. It should be noted that increasing the reaction time may result in the development of the 4-aminophenol diacetylated derivative.
Note that acetic anhydride should be added to the paracetamol aqueous suspension.
The crude solution often appears somewhat yellow or pinkish once the synthesis is completed. The quantitative recovery of the product necessitates excellent filtration and washing with cold water.
The solution can be coloured during the heat dissolving of paracetamol for crystallisation, and the application of activated charcoal does not significantly improve the condition. Slow crystallisation and careful washing of the crystals, on the other hand, result in pearly crystals with a high melting point. Additionally, 4-aminophenol can be crystallised before being synthesised.
When the solution cools, the paracetamol crystallises easily. However, if crystallisation does not occur or occurs slowly, it can be encouraged by gently stroking the inside surface of the crystallisation jar with a glass rod. The crystallisation is virtually instantaneous with this choice, however the crystals are very minute
The crystallisation vessel is placed in an ice bath for a few minutes after cooling to room temperature.
Because the crystals are washed with water, they will be placed in an oven at the proper temperature and/or stored in a desiccator until they reach a constant weight. We consider constant weight a difference of less than 5 mg between two weightings with intermediate drying as a rule, and for the purposes sought. The yields vary between 35 and 70 percent.
If necessary, class time can be cut by using hexane for crystallisation (and crystals washing) (or petroleum ether). This will reduce the time it takes for the product to dry to a consistent weight. In this case, it's important to note that first-year students will find it more difficult to accomplish the crystallisation because the volatile solvent will evaporate during the process. However, if this is the case, students should be encouraged to consider what they will do with the solvent, which must be collected in a clearly labelled non-halogenated organic solvent container for later treatment and recovery.
TLC, m.p., and IR spectra yields are recorded, and the results are explained. The purity and structure elucidation of the product can also be examined using 1 H- and 13C-NMR spectra if the student's background enables it.
It's possible to speculate if the reaction was complete or if the diacetylated derivative was generated if the paracetamol m.p. Is unsatisfactory (169-170.5 oC). To purify a product containing the diacetylated derivative, dissolve the crystals in 10% NaOH (v/v) and reprecipitate with 10% HCl (v/v).
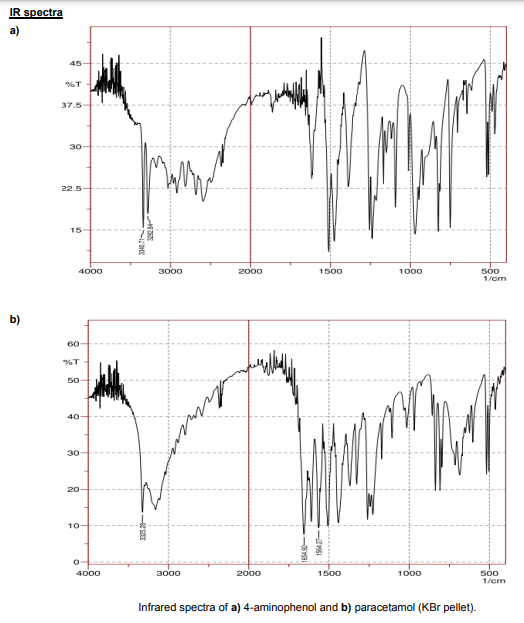
An IR Affinity-1 Shimadzu spectrophotometer was used to gather the IR spectra (KBr pellet). The two N-H amine bands at 3340 and 3282 cm-1 emerge from the bandwidth of the phenolic OH in the IR spectra of 4-aminophenol. The N-H amide band appears approximately 3325 cm-1 in the IR spectrum of paracetamol, despite being on top of the large phenolic O-H band to its right. The appearance of the amide carbonyl band at 1654 cm-1 and the N-H band at 1564 cm-1 are also crucial information bands.
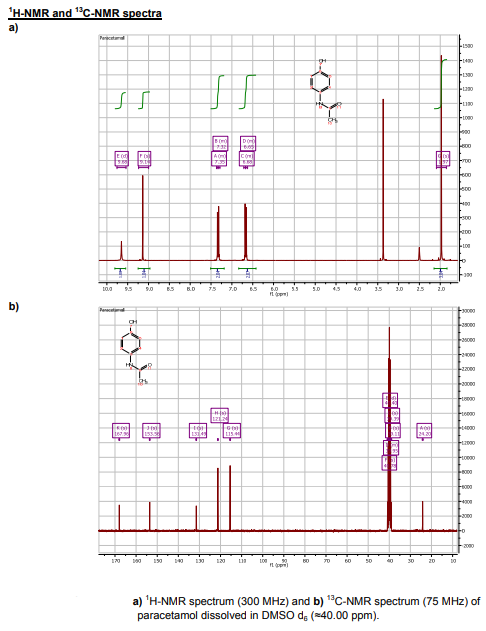
The 1 H-NMR spectrum of paracetamol reveals signals with chemical shifts that are consistent with the suggested structure and published data. The four signals in the aromatic region point to a 1,4-substituted aromatic ring with two distinct substituents: two up fielded singlets of the NH (=9.68 ppm) and OH (=9.14 ppm), and two down fielded ortho-coupled doublets of the aromatic protons at =7.35 and =6.68 ppm. The methyl protons are represented by a big singlet that integrates for three protons at 1.97 ppm. In the spectra reported, DMSO (=3.4 ppm) and water contamination (=2.5 ppm) may also be seen.
The aromatic portion of paracetamol's C-NMR spectrum reveals four signals: one C-OH at 153.56 ppm, one C-NH at 131.49 ppm, and two pairs of comparable C-H. (121.24 and 115.44 ppm). There is also a deshielded carbonyl carbon at 167.96 ppm and a methyl carbon at 24.20 ppm.

Key takeaways:
- Paracetamol is a common and frequently used drug for the treatment of fever (antipyretic) and pain (analgesic) (analgesic). The gastrointestinal tract absorbs paracetamol quickly and practically entirely.
- Your medication's name is Paracetamol 500mg Tablets (called paracetamol throughout this leaflet). The active ingredient in this drug is paracetamol. It is an analgesic (painkiller) that is used to treat pain (such as headaches, toothaches, back pain, and period pain) as well as cold and flu symptoms.
4.4.1 Analgesics:
Analgesics are a type of drug that is used to ease pain. Acetaminophen (Tylenol), which is accessible over the counter (OTC) or by prescription when taken with another drug, and opioids (narcotics), which are exclusively available via prescription, are two examples. Opioids are classified as either conventional or atypical. They have diverse effects on the body.
For extra pain relief, some medications mix acetaminophen with an opioid. However, two opioids should never be taken at the same time.
The use of opioids for persistent pain that isn't caused by cancer is debatable. However, for those with uncontrolled arthritic pain who are unable to use nonsteroidal anti-inflammatory medicines, the medications remain an important therapy choice (NSAIDs). Opioids are closely regulated due to the risk of negative effects and accidental overdose.
4.4.2 What is ibuprofen?
Ibuprofen is an NSAID, or nonsteroidal anti-inflammatory drug, that has analgesic, fever-reducing, and anti-inflammatory actions in larger dosages.
Ibuprofen is listed as an essential drug by the World Health Organization (WHO)Trusted Source. The list identifies the bare minimum of medical requirements for a basic healthcare system.
Steroids and narcotics, or opioids, are two other sorts of pain relievers. Long-term steroid use can have serious side effects, and using opioids can lead to misuse. NSAIDs are safer than both of these
NSAIDs such as ibuprofen, aspirin, and naproxen are well-known, in part because they are available over-the-counter at pharmacies.
By inhibiting the production of cyclooxygenase (COX)-1 and COX-2, ibuprofen lowers pain, fever, edoema, and inflammation. These compounds are released by the body in reaction to illness and injury.
When taking ibuprofen by mouth, the effects should be noticeable after 20–30 minutes.
4.4.3 Ibuprofen Synthesis:
Ibuprofen was created using isobutylbenzene as a starting material. Friedel-Crafts acylation, reduction, chloride substitution, and the Grignard reaction were all used in the synthetic process. IR and 1H NMR spectroscopy were used to examine the products of each step, and melting point analysis was used to confirm the final product.
A five-step ibuprofen synthesis that resembles the industrial BHC synthesis Our synthesis started with a Friedel-Crafts acylation and carbonyl reduction, same as the BHC technique. However, because the commercial technique, which uses carbon monoxide (CO) at 500 psi, was avoided due to safety concerns, we used a chloride substitution, Grignard formation, and Grignard reaction in the last steps.
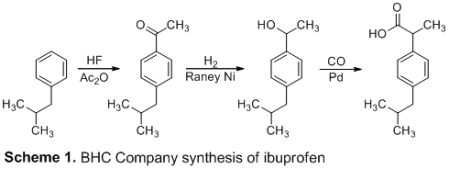
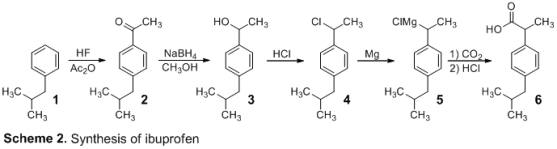
The synthesis of ibuprofen was carried out in a five-step procedure depicted in Scheme 2. To make p-isobutylacetophenone, isobutylbenzene (1) and acetic anhydride were first reacted under Friedel-Crafts acylation conditions (2). The lewis acid combination generated by acetic anhydride and AlCl3 created an acylinium ion, which was subsequently attacked by 1 to make p-isobutylacetophenone (2) by electrophilic aromatic substitution. This product was obtained with a yield of 25.6 percent. IR and 1H NMR spectroscopy were used to investigate Ketone 2. Csp2-H, Csp3-H, C=O, and C=C frequencies were detected in the IR spectra, with maxima at 3026, 2954 cm, 1684, and 1605 cm-1, respectively. The appearance of the relevant ketone functional group can be seen here. The structure of the product was confirmed by the 1H NMR spectra. At 7.89 and 7.15 ppm, the two doublets integrating to two hydrogens suggest four aryl hydrogens that are ortho and meta to the benzene ring's acetyl group. The acetyl group's -hydrogens are indicated by the singlet at 2.52 ppm. The peak at 2.46 ppm indicates the presence of two methylene hydrogens, whereas the peak at 1.88 ppm indicates the presence of a tertiary hydrogen. The signal at 0.91 ppm indicates the presence of six methyl hydrogens. However, there was a large concentration of unreacted isobutylbenzene, which hampered the experiment in subsequent steps. The ketone 2 was reduced with NaBH4 in CH3OH to create 3 in the second phase. Because of the impure starting material from the first reaction, the product was obtained with a yield of 6.8%. The addition of a benzylic hydrogen was validated by 1H NMR spectroscopy, which revealed a new quartet at 4.81 ppm, suggesting the presence of a benzylic hydrogen. Following that, under acidic circumstances, chlorine was replaced for alcohol 3 to create 4 via an SN1 process. The reaction went off without a hitch, yielding 49.2 percent. IR and 1H NMR spectroscopy were used to examine the product. The hydroxyl hydrogen at 1.45 ppm is not visible in the 1H NMR spectra of 4, and the benzylic hydrogen peak has shifted from 4.81 ppm to 5.09 ppm. The broad O-H peak is missing from the IR spectrum, indicating that the alcohol group has been replaced. By reacting 4 with magnesium in refluxing ether, the Grignard reagent, 5, was created. Carbon dioxide was bubbled across 5, allowing the Grignard reagent to attack CO2 nucleophilically and create 6 before protonation. The output of the final ibuprofen product, 6, was 24.6 percent. IR and 1H NMR spectroscopy were used to confirm the expected product. The benzylic hydrogen peak in the 1H NMR spectra of 6 shifted from 5.09 ppm to 3.73 ppm. With new peaks at 1706 cm-1 indicating the carbonyl bond and a broad peak running from 3300 cm-1 to 2400 cm-1 indicating the O-H bond, the IR spectra reveals the creation of the carboxylic acid. Finally, the reported melting point (68–69 °C) is similar to ibuprofen's published melting point3 (75–78 °C), indicating impure ibuprofen production. The overall yield of the synthesis was 1.74 percent prior to the loss of product in step 2. The high amounts of unreacted isobutylbenzene in the first step are largely to blame for the low yield. Allowing for a longer reaction duration or adding a catalyst could increase purity. After obtaining additional stock product, the remaining synthesis produced a 12.1 percent ibuprofen product.
Key takeaways:
Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that can be purchased without a prescription at pharmacies. It is useful in the treatment of pain and fever. Ibuprofen comes with a warning that it can cause serious side effects. Before using this or any other drug, always follow the doctor's recommendations and read the information on the package carefully.
Medical Definition of Antimalarial:
Antimalarial is a term used to describe a medicine that is used to treat malaria. Quinine was the first antimalarial drug, and its name comes from the Peruvian Indian word "kina," which means "tree bark." Quinine, the most significant alkaloid found in cinchona bark, is a huge and complicated molecule. It was the sole effective therapy for malaria until World War I. In fact, quinine was the first chemical molecule to be used to treat an infectious disease successfully.
J.B. Caventou and P.J. Pelletier isolated quinine in crystalline form in 1820. R.B. Woodward and W. Doering synthesised quinine for the first time in 1944, making it one of the classic successes of synthetic organic chemistry.
Quinine works by preventing Plasmodium, the malarial parasite that resides inside the victim's red blood cells, from growing and reproducing. Quinine enables the parasites to vanish from the bloodstream, alleviating the disease's symptoms. Many individuals, however, relapse after quinine treatment has ended. They contract malaria again because quinine fails to eliminate malarial parasites in cells other than red blood cells. These parasites remain, and after a period of time, they reinvade red blood cells, causing a relapse.
Better medications were sought because quinine does not treat malaria permanently. Several substitutes for quinine were discovered during and after WWII. Some of these medications (such as chloroquine and chloroguanide) are more effective than quinine at stopping malarial parasites from multiplying in the bloodstream. Others, such as primaquine and pyrimethamine, act on the parasite's blood and tissue phases, resulting in a complete cure and preventing relapse. Quinine was originally used to alleviate leg cramps, but due to dangerous adverse effects such as low platelet counts and mortality, it is no longer FDA approved for this usage.
Structure:
What is Chloramphenicol and How Does it Work?
For the treatment of systemic infections and severe illnesses
Chloramphenicol is sold under several brand names, including Chloramphenicol IV and Chloromycetin. In the United States, these brand names have been phased out. Chloramphenicol Doses:
Injectable remedy
Each vial contains 1,000 mg
Considerations for Dosage - Give as follows:
Susceptible Strains Cause Serious Infections
Those who are adults:
50 mg/kg/day intravenously divided every 6 hours; in rare circumstances, patients with moderately resistant organisms or severe infections may need a higher dose of up to 100 mg/kg/day; reduce these high dosages as soon as possible.
Infections that affect the entire body
Children's health:
Infants and children: When appropriate cerebrospinal fluid concentrations are sought, up to 100 mg/kg/day may be required; however, dose should be reduced to 50 mg/kg/day as soon as possible.
Infants and children with suspected immature metabolic functions: 25 mg/kg/day divided every 6 hours should result in therapeutic medication concentrations in the blood.
Neonates are children who are born prematurely (Infants younger than 28 days
Children's health
Loading dosage (LdD): 20 mg/kg intravenously once; maintenance dosage should be given 12 hours following the loading dose.
Dose for Maintenance
Infants under the age of 7 days: 25 mg/kg intravenously every 24 hours.
Infants weighing less than 2000 g and older than 7 days: 25 mg/kg/day intravenously every 24 hours
Infants over 7 days old and weighing more than 2000 g: 50 mg/kg/day administered intravenously every 12 hours
Additional Information
Children's health
Troughs 5-10 mg/l, peaks 10-20 mg/l
Other Indications and Applications
Those who are adults:
Only use as a last resort for meningitis, typhoid, or rickettsial infection.
What Other Drugs Interact with Chloramphenicol?
If your doctor has prescribed this medication, your doctor or pharmacist may already be aware of any potential drug interactions and is keeping an eye on you. Before starting, stopping, or changing the dosage of any prescription, consult your doctor, health care provider, or pharmacist.
There are no documented serious medication interactions with chloramphenicol.
Chloramphenicol has a number of serious side effects, including:
Lurasidone is a kind of lurasidone that is used
Chloramphenicol has a number of serious side effects, including
Live BCG vaccination
Cefoxitin is a kind of cefoxitin.
Vaccination against cholera
Mefloquine is a drug that is used to treat malaria.
Rice with red yeast
Live typhoid vaccine
Vilazodone is a kind of vilazodone that is used to
Anticoagulant (warfarin)
Chloramphenicol has a number of moderate interactions, including:
Axitinib is a drug that is used to treat cancer.
Conjugated estrogens/bazedoxifene
Ceftriaxone is a cephalosporin antibiotic.
Vaginal estrogens, conjugated estrogens
Eluxadoline fluradoline eluxadoline
Estradiol is a kind of oestrogen.
Estrogens that have been conjugated synthetically
Estrogens at have been esterified
Estropipate estropi
Fosphenytoin
Ivacoftor
Lomitapide is a kind of lomitapide.
Maraviroc.com
Mestranol is a kind of mestranol.
Ospemifene
Phenytoin (phenytoin)
Piperacillin is a kind of antibiotic.
Anhydrous citric acid/magnesium oxide/sodium picosulfate
Chloramphenicol interacts with 47 distinct medications in modest ways.
This list does not include all potential interactions or side effects. As a result, inform your doctor or pharmacist of all the products you use before using this product. Keep a list of all your prescriptions on you at all times, and share it with your doctor and pharmacist. If you have any health issues, concerns, or would like more information about this medicine, consult your health care provider or doctor.
4.7.1 Scientific Health Benefits of Curcumin:
Curcumin Is an Anti-Inflammatory
Turmeric's greatest claim to fame is that it's often used to combat inflammation, and curcumin is responsible for the majority of turmeric's anti-inflammatory properties. According to a previous study, curcumin may be a more effective anti-inflammatory treatment than conventional anti-inflammatory drugs like Advil (ibuprofen) and aspirin in the proper amount.
Curcumin may aid in the treatment of inflammatory bowel disease, pancreatitis, and arthritis, as chronic inflammation plays a role in many chronic diseases. Later, we'll go through some of the specific advantages.
Curcumin May Protect Against Heart Disease
A past study shows that curcumin may improve endothelial function, or the health of the thin membrane that covers the inside of the heart and blood vessels. This membrane plays a key role in regulating blood pressure. Lower endothelial function is associated with aging and an increased risk of heart disease. Thus, curcumin may help protect against age-related loss of function and reduce your likelihood of developing heart disease.
In one study, researchers compared the effects of an eight-week aerobic exercise program and a curcumin supplement in improving endothelial function in postmenopausal women. Both the exercise and the curcumin group saw equal improvements in endothelial function, whereas the control group saw no changes.
Another study found that curcumin was equally effective at improving endothelial function in people with type 2 diabetes (heart disease is a common comorbidity of type 2) as the drug Lipitor (atorvastatin), a medication commonly prescribed to reduce the risk of heart attack and stroke.
Still, more research is needed to determine if curcumin is a safe and effective long-term treatment strategy for people with heart disease.
Curcumin May Prevent (and Possibly Help Treat) Cancer
As inflammation is linked to tumor growth, anti-inflammatory compounds such as curcumin may play a role in treating and preventing a variety of cancer types, including colorectal, pancreatic, prostate, breast, and gastric cancers. In fact, research in mice suggests that curcumin may help slow the spread of tumor cells and may even prevent tumors from forming in the first place. It may do this in several ways, including disrupting the formation of cancerous cells at various stages in the cell cycle, interfering with cell signaling pathways, and even causing those cancerous cells to die.
Whether curcumin can help treat cancer in humans has yet to be determined, but the research is ongoing.
Curcumin May Help Ease Symptoms of Osteoarthritis
Curcumin may be a safe and effective long-term therapeutic option for persons with osteoarthritis due to its significant anti-inflammatory characteristics (OA). In a previous trial, patients with osteoarthritis who took 1,000 mg of Meriva daily for eight months reported significant improvements in stiffness and physical function, whereas the control group had no changes. Meriva is a patented treatment that contains a natural curcuminoid mixture (75% curcumin, 15% demethoxycurcumin, and 10% bisdemethoxycurcumin), phosphatidylcholine (a substance present in eggs, soybeans, and other foods), and microcrystalline cellulose (a refined wood pulp commonly used by the pharmaceutical and food industries).
In a mouse study published in the June 2016 issue of Arthritis Research & Therapy, researchers discovered that 50 mg oral curcumin per kilogramme (kg) body weight considerably reduced the course of OA, while a topical curcumin treatment offered pain alleviation. However, it has to be established if these advantages would extend to humans.
Curcumin May Help Treat or Prevent Diabetes
Curcumin may help treat and prevent diabetes, as well as related conditions including diabetic nephropathy (commonly known as diabetic kidney disease), which affects persons with both type 1 and type 2 diabetes, according to a previous evaluation of studies. One disadvantage is that many of the research were conducted on animals rather than humans.
One study reported that feeding rats with type 2 diabetes 80 mg of tetrahydrocurcumin (one of the primary compounds in curcumin) per kg body weight for 45 days resulted in a significant decrease in blood sugar and a rise in plasma insulin.
Curcumin supplements helped obese mice with type 2 diabetes lower blood insulin levels after 16 weeks, according to a study published in the July 2019 issue of Nutrition & Metabolism.
Meanwhile, curcumin's anti-inflammatory and antioxidant properties may help prevent diabetes and improve many of the factors that contribute to diabetes, such as insulin resistance, high blood sugar, and hyperlipidemia (an elevated level of fat in the blood; one type of hyperlipidemia is characterised by high levels of LDL, or "bad," cholesterol). To be sure, more human research are needed.
Curcumin May Play a Role in Treating Rheumatoid Arthritis
Curcumin has shown promise as a treatment for rheumatoid arthritis (RA), a chronic inflammatory disease that affects the joints but can also damage the eyes, lungs, skin, heart, and blood vessels. RA produces severe swelling of the joints, which can destroy the bones over time, resulting in deformities and physical limitations.
Patients with RA were given 500 mg of curcumin, 50 mg of diclofenac sodium (a prescription nonsteroidal anti-inflammatory medication), or a combination of the two in one research. When compared to the other two groups, the curcumin-only group exhibited significant improvements in joint discomfort and swelling after eight weeks. The curcumin treatment was likewise shown to be safe, with no adverse effects.
Curcumin May Prevent Eye Degeneration
Glaucoma is a category of eye diseases that causes blindness in persons over the age of 60. Unfortunately, your vision cannot be restored after it has been lost.
However, preliminary study published in Scientific Reports in July 2018 suggests that topical curcumin therapies may help protect the eyes from degeneration. For three weeks, researchers gave rats a customised curcumin eye drop solution twice a day. When compared to the treatment group, the untreated rats had a 23 percent reduction in retinal cells by the end of the trial, indicating that the curcumin treatment prevented loss. The outcomes of the study are promising, but additional research is needed to see if curcumin can prevent eye deterioration in humans.
Key takeaways:
- Curcumin can aid with oxidative and inflammatory disorders, metabolic syndrome, arthritis, anxiety, and hyperlipidemia, among other things. It may also aid in the treatment of exercise-induced inflammation and muscular pain, allowing active persons to recover faster and perform better.
- Long-term use of high doses of turmeric and curcumin is not recommended due to a lack of data establishing their safety. The World Health Organization (WHO) has concluded that a daily dose of 1.4 mg per pound (0–3 mg/kg) of body weight is acceptable (18).
4.8.1 Azadirachtin:
Azadirachtin is a group of compounds. It is a mixture of related substances extracted from neem seed kernels with a complex structure. Azadirachtin is only found in the seeds. Azadirachtin has a variety of effects on insects, including serving as a growth regulator, anti-feedant, repellant, sterilant, and oviposition inhibitor.
By suppressing the synthesis or metabolism of the insect moulting hormone ecdysone, azadirachtin functions as an antagonist as an insect growth regulator. As a result of slowing the moulting process and subsequent metamorphosis, insects perish when transitioning to the next life stage or instar (insect stage between moults), breaking the insect life cycle and preventing future generations from being produced. Azadirachtin is more effective on insects in their immature/young life stages than on eggs or adults. However, azadirachtin takes longer to work than other pesticides, owing to the fact that it alters or modifies insect behaviour. Azadirachtin is a stomach poison that requires insects to absorb the active ingredient while feeding in order to be poisoned. Chewing insects seemed to respond better to activity than sucking insects. This could explain why azadirachtin works so well against caterpillars. Most insect pests have modest contact action with azadirachtin, and it works best at warmer temperatures (>70 F), with lesser efficacy at lower temperatures. Azadirachtin has been demonstrated to exhibit systemic qualities, including activity against specific insect pests, albeit this is dependent on the plant type and pH of the growing medium, with reduced systemic activity at pH levels higher than 7.0. (alkaline). This is intriguing because azadirachtin is water insoluble (0.05 ppm). Furthermore, azadirachtin foliar sprays have been shown to be beneficial in decreasing populations of the two-spotted spider mite, Tetranychus urticae, in some experiments. Azatin, Ornazin, AzaGuard, Molt-X, Azatrol, AzaSol, and Aza-Direct are just a few of the products using azadirachtin as the active ingredient that have been approved for use in greenhouses. Aphids, caterpillars, leaf miners, mealybugs, scales, thrips, and whiteflies are all targets for the majority of these products. The hydrophobic extract of neem oil has been clarified. Insect and mite pests are suffocated (breathing passages are blocked) by a clarified hydrophobic extract of neem oil (neem oil). Aphids, whiteflies, spider mites, mealybugs, and scales are among the soft-bodied insect and mite pests that neem oil is particularly effective against. Eggs, immatures (larvae or nymphs), and adults may be killed by neem oil. Because neem oil only has contact activity, it's critical to get a good coverage of all plant parts and to apply it again according to the label's instructions. Triact is the only product with a refined hydrophobic extract of neem oil as the active ingredient that is approved for use in greenhouses. Aphids, leafhoppers, mealybugs, mites, scales, and whiteflies are all targets for this product. Due to its vulnerability to ultra-violet light (sunlight) degradation, azadirachtin and purified hydrophobic extract of neem oil have short residual action, which necessitates repeated applications. Both compounds have a low toxicity to humans and mammals, with LD50 values of more than 5,000 mg/kg. Furthermore, azadirachtin and the clarified hydrophobic extract of neem oil are less toxic to most natural enemies (parasitoids and predators) than traditional insecticides.
4.8.2 Therapeutics Role of Azadirachta indica (Neem).
Botanical Description of Neem
The Meliaceae family includes the neem tree, which is abundant in tropical and semitropical regions such as India, Bangladesh, Pakistan, and Nepal. It is a fast-growing tree that reaches a height of 20–23 metres and has a straight trunk with a diameter of 4-5 feet. The leaves are complex, imparipinnate, and have 5–15 leaflets each. It bears green drupes that develop to a golden yellow colour in the months of June–August. Table 1 shows the taxonomic classification of Azadirachta indica (neem).
Table:
Taxonomic position of Azadirachtaindica (neem)
Order | Rutales |
Suborder | Rutinae |
Family Meliaceae |
|
Subfamily | Meliodeae |
Tribe | Melieae |
Genus | Azadirachta |
Species | Indica |
Active Compounds of Azadirachta indica L. (Neem)
Because of its abundant source of numerous sorts of components, Azadirachta indica L. (neem) has a therapeutic role in health management. Azadirachtin is the most active ingredient, followed by nimbolinin, nimbin, nimbidin, nimbidol, sodium nimbinate, gedunin, salannin, and quercetin. Nimbin, nimbanene, 6-desacetylnimbinene, nimbandiol, nimbolide, ascorbic acid, n-hexacosanol and amino acid, 7-desacetyl-7-benzoylazadiradione, 17-hydroxyazadiradione, and nimbiol are all found in the leaves [15–17]. Polyphenolic flavonoids quercetin and ß-sitosterol were isolated from neem fresh leaves and were known to have antibacterial and antifungal activities [6], and seeds contain important compounds such as gedunin and azadirachtin.
Mechanism of Action of Active Compounds
Neem (Azadirachta indica), a member of the Meliaceae family, has therapeutics implication in the disease’s prevention and treatment. But the exact molecular mechanism in the prevention of pathogenesis is not understood entirely. It is considered that Azadirachta indica shows therapeutic role due to the rich source of antioxidant and other valuable active compounds such as azadirachtin, nimbolinin, nimbin, nimbidin, nimbidol, salannin, and quercetin.
Possible mechanism of action of Azadirachta indica is presented as follows.
Neem (Azadirachta indica) plants parts shows antimicrobial role through inhibitory effect on microbial growth/potentiality of cell wall breakdown. Azadirachtin, a complex tetranortriterpenoid limonoid present in seeds, is the key constituent responsible for both antifeedant and toxic effects in insects [18]. Results suggest that the ethanol extract of neem leaves showed in vitro antibacterial activity against both Staphylococcus aureus and MRSA with greatest zones of inhibition noted at 100% concentration [19].
- Neem plays role as free radical scavenging properties due to rich source of antioxidant. Azadirachtin and nimbolide showed concentration-dependent antiradical scavenging activity and reductive potential in the following order: nimbolide > azadirachtin > ascorbate [20].
- Neem ingredient shows effective role in the management of cancer through the regulation of cell signaling pathways. Neem modulates the activity of various tumour suppressor genes (e.g., p53, pTEN), angiogenesis (VEGF), transcription factors (e.g., NF-κB), and apoptosis (e.g., bcl2, bax).
- Neem also plays role as anti-inflammatory via regulation of proinflammatory enzyme activities including cyclooxygenase (COX), and lipoxygenase (LOX) enzyme.
Therapeutic Implications of Neem and Its Various Ingredients in Health Management
Active constituents help to heal diseases by activating antioxidative enzymes, rupturing bacteria's cell walls, and acting as a chemopreventive via regulating cellular pathways. The pharmacological properties of neem are thoroughly examined.
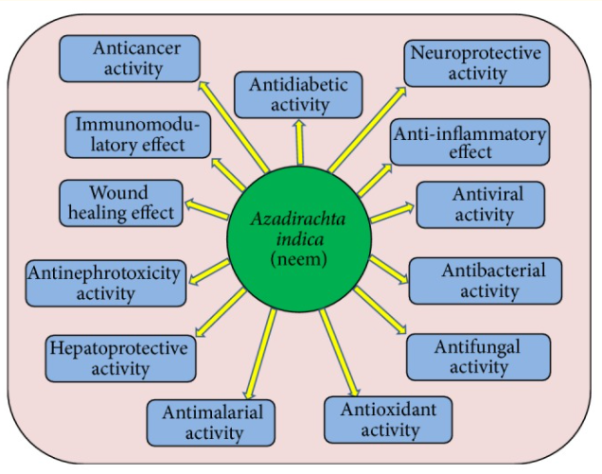
Azadirachta indica L. Neem pharmacological activities in disease management through regulation of different processes
Antioxidant Activity
One of the main causes of disease is free radicals, also known as reactive oxygen species. The neutralisation of free radical activity, on the other hand, is a key step in disease prevention. Antioxidants help to stabilise and deactivate free radicals before they assault targets in biological cells, and they also help to activate antioxidative enzymes that help to regulate the damage produced by free radicals and reactive oxygen species. Antioxidant activity has been reported in medicinal plants. Because of their high antioxidant content, plants' fruits, seeds, oil, leaves, bark, and roots play a significant role in disease prevention.
The antioxidant activity of A. Indica leaf and bark extracts was investigated, and the results showed that all of the examined leaf and bark extracts/fractions of neem cultivated in the foothills have strong antioxidant capabilities. Another key study looked at the antioxidant activity of leaves, fruits, flowers, and stem bark extracts from the Siamese neem tree, and the results showed that extracts from the leaves, flowers, and stem bark contain a lot of antioxidant potential.
The following study was conducted to examine in vitro antioxidant activity in different crude extracts of Azadirachta indica (neem) leaves, as well as antioxidant capacity of different crude extracts: methanol extract > chloroform extract > butanol extract > ethyl acetate extract > hexane extract > methanol extract The new discovery suggests that chloroform crude neem extracts could be employed as a natural antioxidant.
Other findings revealed that azadirachtin and nimbolide, in the sequence nimbolide > azadirachtin > ascorbate, had concentration-dependent antiradical scavenging activity and reductive potential. Additionally, azadirachtin and nimbolide treatment prevented the formation of DMBA-induced HBP carcinomas by preventing procarcinogen activation and oxidative DNA damage, as well as upregulating antioxidant and carcinogen detoxification enzymes. The antioxidant activity of the flowers and seed oil of the neem plant Azadirachta indica A. Juss. Was tested, and the results showed that the ethanolic extract of the flowers and seed oil at 200 g/mL produced the highest free radical scavenging activity, with 64.17 0.02 percent and 66.34 0.06 percent, respectively.
Root bark extract had a stronger free radical scavenging impact, with 50 percent scavenging activity at 27.3 g/mL, and total antioxidant activity of 0.58 mM of standard ascorbic acid, according to the study's findings. According to the study's findings, neem leaf and bark extracts/fractions grown in the foothills (subtropical zone) exhibit substantial antioxidant effects.
The antioxidant activity of leaves, fruits, flowers, and stem bark extracts from the Siamese neem tree was tested, and the results revealed that leaf aqueous extract, flower, and stem bark ethanol extracts had higher free radical scavenging activity with 50 percent scavenging activity at 26.5, 27.9, and 30.6 microg/mL, respectively. Furthermore, the total antioxidant activity of the extracts was found to be 0.959, 0.988, and 1.064 mM, respectively, of standard trolox.
Anti-cancerous Activity
Cancer is a complex disease that affects people all around the world. Changes in molecular/genetic pathways play a role in cancer's growth and progression. The allopathic therapy module is effective on one hand, but it has a negative impact on normal cells. Plants and their contents have previously been shown to prevent the growth of malignant cells through modulating cellular proliferation, apoptosis, tumour suppressor genes, and a variety of other molecular pathways. Flavonoids and other compounds in neem help to prevent cancer by inhibiting the growth of cancer cells (Figure). A large number of epidemiological studies suggest that a high flavonoid consumption is linked to a lower cancer risk.
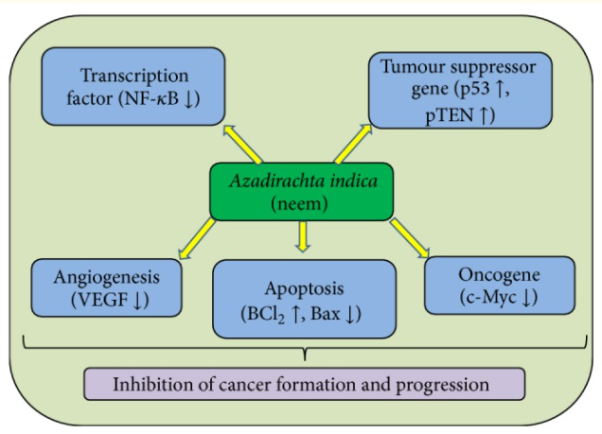
Anti-cancerous activities of Azadirachta indica L. Neem through the modulation of various cell signaling pathways.
Neem oil contains different neem limonoids, which reduce 7,12-dimethylbenz(a)anthracene's mutagenesis effects. The cytotoxic effects of nimbolide found in leaves and flowers on human choriocarcinoma (BeWo) cells were investigated in this study, and the results showed that treatment with nimbolide resulted in dose- and time-dependent inhibition of BeWo cell growth, with IC50 values of 2.01 and 1.19 M for 7 and 24 hours, respectively. The chemo preventive potential of the limonoids, azadirachtin, and nimbolide was investigated, and the findings revealed that azadirachtin and nimbolide inhibited the development of DMBA-induced HBP carcinomas by influencing multiple mechanisms, including prevention of procarcinogen activation and oxidative DNA damage, upregulation of antioxidant and carcinogen detoxification enzymes, and inhibition of DMBA-induced HBP carcinoma
Azadirachta indica and its active chemicals have an important role in cancer prevention and progression. The actual chemical process at work in this vision is unknown. Neem and its components are thought to play a function in the modulation of numerous cells signalling pathways, according to research. Numerous compounds of Azadirachta indica activate tumour suppressor genes and inactivate the activity of several genes associated in cancer formation and progression, such as VEGF, NF-B, and PI3K/Akt. Neem has been shown to be a good tumour suppressor gene activator as well as an inhibitor of the VEGF and phosphoinositol PI3K/Akt pathways. It also stimulates apoptosis, inhibits NF-B signalling, and activates the cyclooxygenase pathway.
Through the modification of molecular pathways mentioned below, neem and its constituents play a role in the prevention of cancers.
Key takeaways:
- Neem is POSSIBLY UNSAFE when taken by mouth in large doses or for long periods of time. It might harm the kidneys and liver.
- When applied to the skin: Neem leaf extract gel is POSSIBLY SAFE when applied inside the mouth for up to 6 weeks. Neem oil or cream is POSSIBLY SAFE when applied to the skin for up to 2 weeks.
- The pesticide action of neem oil is attributed to azadirachtin, which is used as an insecticide for arthropod pests. Poisoning causes vomiting within minutes to hours, followed by drowsiness, tachypnea, and generalized seizures associated with loss of consciousness and coma.
Drug Interactions between ranitidine and Vitamin C:
Ranitidine
A total of 132 drugs are known to interact with ranitidine.
- Ranitidine is in the drug class H2 antagonists.
- Ranitidine is used to treat the following conditions:
- Cutaneous Mastocytosis
- Duodenal Ulcer
- Duodenal Ulcer Prophylaxis
- Eczema
- Erosive Esophagitis
- Gastric Ulcer Maintenance Treatment
- Gastrointestinal Hemorrhage
- GERD
- Hiatal Hernia
- Indigestion
- Laryngopharyngeal Reflux
- Pathological Hypersecretory Conditions
- Stomach Ulcer
- Stress Ulcer Prophylaxis
- Surgical Prophylaxis
- Urticaria
- Zollinger-Ellison Syndrome
Why it’s used
Ranitidine oral tablet is used to treat several conditions, including:
- Intestinal and stomach ulcers
- Gastroesophageal reflux disease (GERD)
- Erosive esophagitis
- Conditions where your stomach makes too much acid, such as Zollinger-Ellison syndrome
Ranitidine may be used as part of a combination therapy. This means you may need to take it with other medications.
Ranitidine is typically used for short-term treatment, especially for GERD. If you’re taking this drug for other conditions, you may need long-term treatment. You may need to take it for several weeks or months.
Ranitidine side effects
Drowsiness and other adverse effects are possible with ranitidine oral tablet.
More common side effects
The more common side effects of ranitidine oral tablet can include:
- Headache
- Constipation
- Diarrhoea
- Nausea and vomiting
- Stomach discomfort or pain
If these effects are mild, they may go away within a few days or a couple of weeks. If they’re more severe or don’t go away, talk to your doctor or pharmacist.
Serious side effects
If you experience any major side effects, contact your doctor straight immediately. If your symptoms are life-threatening or you believe you are having a medical emergency, dial 911. The following are examples of serious side effects and associated symptoms:
• Liver inflammation, which can cause symptoms such as:
o Yellowing of your skin or your eyes' whites
o exhaustion
o Urine that is dark
o Stomach ache
• Changes in brain function, including symptoms like:
o No perplexity
o Upheaval
o Depressed mood
o Visual or auditory hallucinations (seeing or hearing something that isn't there)
o Hazy vision
• Symptoms of an abnormal heart rate include:
o A rapid heart rate
o Exhaustion
o A feeling of being out of breath
Vitamin C
Vitamin C is known to interact with a total of 27 medications.
Vitamin C belongs to the class of drugs known as vitamins.
The following conditions are treated with vitamin C:
1. Dietary Supplementation is the first step.
2. The ailment of scurv
3. Acidification of the Urine
4.10.1 Colour and Constitution:
Visible light is electromagnetic radiation having a rather narrow range of wavelengths (400-800nm). A black substance absorbs all wavelengths of visible light. Selective absorption of visible light by a substance imparts color, but the color is not that of the light absorbed but instead of the residual light that the substance transmits or reflects. For example, a compound that absorbs in the region 435-480nm removes blue light from the visible spectrum, and the residual light is recognized by the eye as being yellow. The relationship of the observed color to wavelength of light absorbed is shown in Table 28-1. It is customary to call the color observed the complementary color or the subtraction color to that absorbed.
Table: Color and wavelength
Light absorbed | Complementary (subtraction) color seen | |
Wavelength(nm) | Color | |
400-435 | Violet | Green-yellow |
435-480 | Blue | Yellow |
480-490 | Green-blue | Orange |
490-5900 | Blue-green (cyan) | Red |
500-560 | Green | Purple(magenta) |
595-605 | Orange | Green-blue |
605-750 | Red | Blue-green (cyan) |
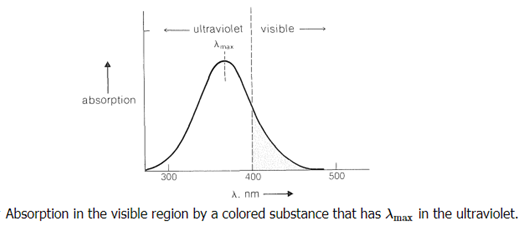
Clearly, the colour perceived, as well as its brightness and intensity, is determined by the form of the absorbing substance's electronic spectral curve, which is determined by the chemical structure of the substance. The energy of the corresponding electronic transitions decreases when absorption shifts from the blue to the red end of the spectrum. We also know that this tendency is linked to an increase in multiple bond conjugation. Colorless 1,2-diphenylethene, for example, is yellow-orange 1,10-diphenyl-1,3,5,7,9-decapentaene:
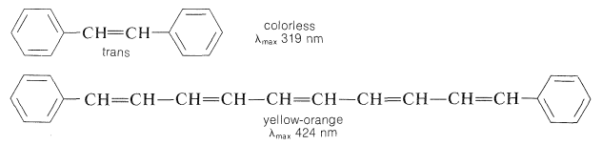
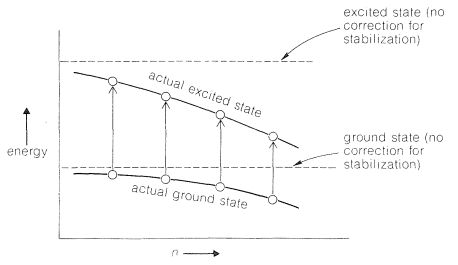
Figure shows how conjugation stabilises both the ground and excited states, but the excited state more so than the ground state. As conjugation increases, the gap between the states narrows, and absorption moves to longer wavelengths (also see Section 9-9B and 21-5C).
Because most dyes have relatively short conjugated systems and would not be brightly coloured in the absence of substituent groups, the effect of substituents on colours associated with conjugated systems is of special interest in dye research. (The plant pigments -carotene, Section 2-1, and lycopene, which is commonly used as a food colouring, are exceptions.)
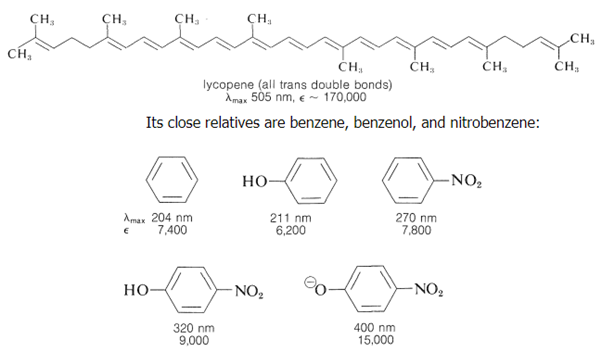
The benzenoid ring, also known as the absorbing chromophore, is the conjugated system that all four molecules share (Section 22-3B). Individually, the hydroxyl and nitro substituents may be seen to move the chromophore's maximum wavelength to longer wavelengths. However, when the OH group is transformed to the equivalent anion, 4-nitrobenzenolate, the combined action of the two substituents is much more significant. Now that max has been relocated into the visible range, the colour has become yellow, and because is enormous, the colour is quite bright. As a result, the major benzenoid absorption band can be shifted from the ultraviolet to the visible portion of the spectrum with the right substituents. Auxochromes are a term used to describe such substituents. They work by prolonging the chromophore's conjugation, and they're especially good at inducing substantial shifts towards the visible when one of the substituents is a -electron donor and the other is a -electron acceptor. The interaction between the strongly electron-donating O group and the strongly electron-accepting NO2 group offers significant stability for the 4-nitrobenzenolate ion:
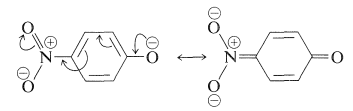
Because excited electronic states feature hybrid structures with significantly more major contributions from dipolar valence-bond forms than the ground state, the energy gap is projected to shrink (see Section 9-9B). Another approach to think about the effect of substituents is to remember that resonance structures like 17a and 17b will help to stabilise excited singlet states of benzene:
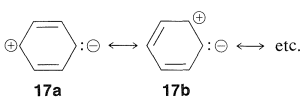
As a result, substituting an electron-attracting group (such as NO2) at one end of such a system for an electron-donating group (such as O) at the other should be especially beneficial for stabilising the excited state (relative to the ground state, where 17a, 17b, etc., are of lesser importance). At the same time, two electron-attracting (or two electron-donating) groups at opposite ends should not be expected to be nearly as effective.
Many strongly coloured natural or synthetic chemicals have conjugated structures with substituents, commonly cationic or anionic substituents, that can donate or take electrons from the conjugated system, as we hope you can see from the preceding explanation. These compounds are used to make a variety of dyes, pigments, indicators, and food colouring agents, as well as giving plants and animals colour. Here are a few examples:
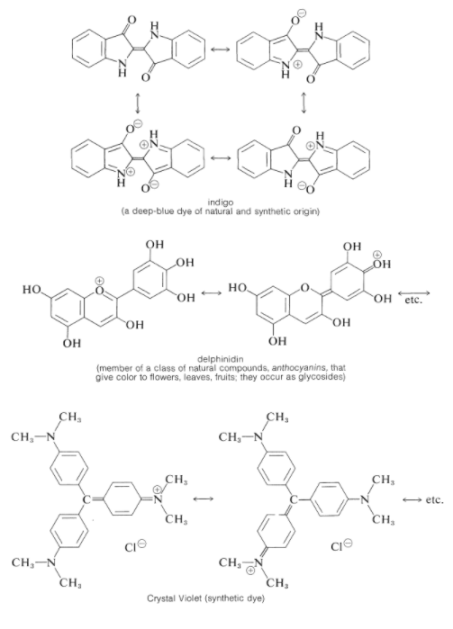
4.10.2 Dyes
The dye industry has long been associated with the advancement of synthetic organic chemistry. Although dyes have been taken from natural sources for ages, a synthetic dye was not commercially created until 1856. At the age of 17, William Henry Perkin oxidised benzenamine (aniline) with potassium dichromate and separated a purple compound that was suitable for dyeing silk from the product (which was primarily aniline black; Section 23-11D). Perkin had a difficult time getting the dye into commercial production. He had to design and build his own equipment as well as invent efficient syntheses for starting materials because there was no organic chemical industry at the time. His path to benzenamine began with coal-derived crude benzene, which he nitrated before reducing with iron and acid. Because concentrated nitric acid was unavailable, he had to produce his own (from nitrate salts and sulfuric acid). Otto Fischer did not discover the structure of Perkin's dye, known as mauveine, until 1890. Because the benzene utilised contained methylbenzene, the dye was actually a mixture), yet the result of benzenamine oxidation is structurally comparable to aniline black:
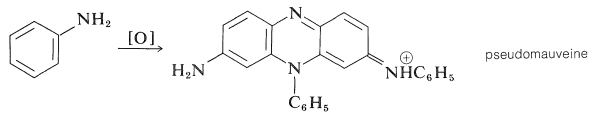
Despite the fact that superior dyes have replaced the mauveine dyes, they are representative of a series of useful dyes with the same general structure.
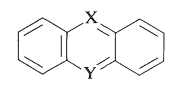
X and Y can be oxygen, nitrogen, sulphur, or carbon, respectively. The rings almost always have substituents (hydroxyl or amino) that help to stabilise the exciting states. The following are some examples of ring systems:
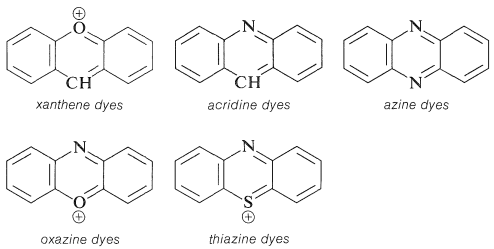
Substituted triphenylmethane derivatives are used to make a variety of useful colours. This type of dye is exemplified by crystal violet (Section 28-4) and phenolphthalein.
Other significant dyes are derived from the chemicals listed below:
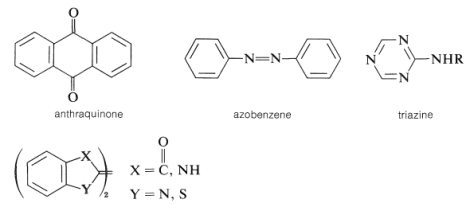
Examples are
There is more to a successful dye than just an attractive color.5 If it is to be useful, say for coloring fabrics, some simple means must be available for introducing the color into the fiber and then, usually of greater difficulty and importance, the color must be reasonably permanent - that is, resistant to normal laundry or cleaning procedures (wash-fast) and stable to light (light-fast). Here again, fundamentally important problems are involved. The scientific approach to improving wash-fastness of fabric dyes has to be based on a knowledge of the structural factors bearing on the intermolecular forces that determine solubilities. Light-fastness is connected with the photochemistry of organic compounds.
4.10.3 Classification of Dye:
Natural Dye
Dying has been a flourishing trade since long, in different parts of the world. The dyes used in times before progress in chemical science were only natural. Dyes were derived from plants and animals. Indigo trade and farming in northern India is an example of the scale of trade.
Synthetic dyes have taken over the industry because of less cost and more reliability but natural dyes such as haematoxylin, carmine and orcein are still in use in the industry.
Synthetic Dye
Synthetic dyes are those that are made from organic and inorganic chemical components. Synthetic dyes include acidic, basic, azoic, nitro, vat dyes, mordant dyes, and sulphur dyes, among others.
Direct Dye
Preparing an aqueous solution and immersing the fabric in it is how these dyes are applied to the fabric. Direct dyes are used to colour fabrics that can form hydrogen bonds with the dye molecule. With the introduction of this method for cotton dying, the use of mordants or other binders became superfluous. Direct dyes are more fastidious than other dyes, but they lack the colour brilliance of other dyes. The diazotization treatment compensates for this. Direct dyes are used to dye fabrics such as cotton, linen, rayon, wool, silk, and nylon.
Disperse Dye
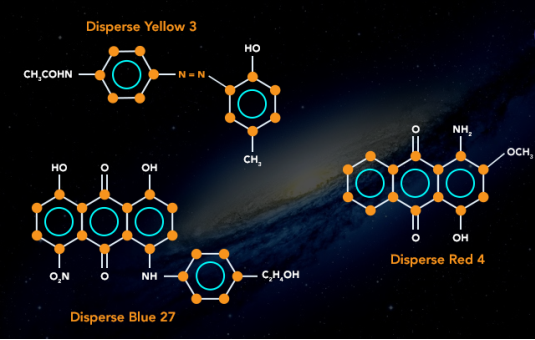
These dyes were created to colour secondary cellular acetate fibres and are relatively water insoluble. Disperse dyes are made by grinding dye into small particles and dissolving it in a solution with the help of dispersing agents. When fibre is submerged in the solution, it absorbs the colour. Disperse dyes are used to colour polyester, nylon, acetate, and triacetate fibres.
Reactive Dye
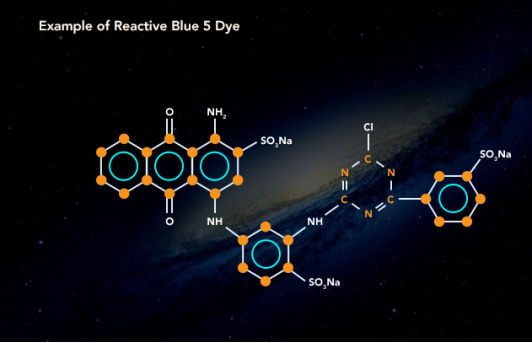
Covalent bonds are created with cellulose fibres in reactive dyes. Such connections have a high degree of fastness. It happens because strong molecular connections are difficult to break. As a result, they have a higher comparative resistance to light and washing. Read on to learn more about the benefits of reactive dyes.
Solvent Dye
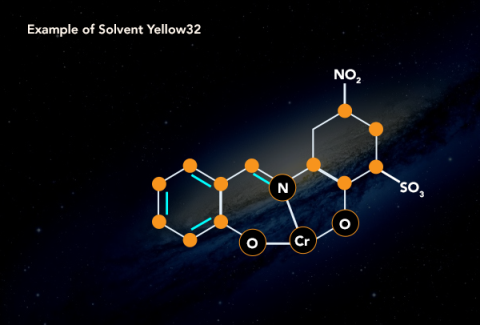
Alcohol, chlorinated hydrocarbons, and liquid ammonia are all soluble in these colours, but not water. These dyes are widely used in the petroleum sector. The dye's colours are created by dissolving it in a target, which is usually lipids or non-polar solvents. Plastics, synthetics, gasoline, oil, and waxes are all coloured with them. Due to the rising industries in emerging countries, all types of dyes are in high demand. As a developing country, India's future market trends are encouraging. The recent trade wars between the United States and China have provided India with an opportunity to expand its domestic market by serving clients from the Asia Pacific area. As a result, India's dyes manufacturers anticipate increased market growth. India Dyes Market Outlook to 2022 has been published by ResearchAndMarkets.com. Market Structure (Organized and Unorganized), Product Type (Reactive, Disperse, Direct, Acid, VAT, and Others), and Applications (Textiles, Leather, Printing Inks, Paper and Others).
4.11.1 Mordant dye:
Mordant dye is a colourant that can be bonded to a material for which it has little or no affinity without the use of a mordant, which is a chemical that mixes with the dye and the fibre. Because dichromates and chromium complexes are the most common modern mordants, the term "mordant dye" usually refers to chrome dye. With different mordants, most mordant dyes produce distinct colours. Wool, wool blends, silk, cotton, and certain modified-cellulose fibres can all be dyed with mordant dyes.
The colours that Alizarin produces in the presence of various ions are listed below.
Ions and colours
Al3+ = Rose red
Ba 2+ = Blue
Cr 3- = Brownish red
Mg 2+ = Violet
Sr 2+ = Red
4.11.2 Vat dye:
Vat dye is a broad category of water-insoluble dyes, including indigo and anthraquinone derivatives, that are employed mostly on cellulosic fibres. To impregnate the fibre, the dye is administered in a soluble, reduced form, which is then oxidised in the fibre back to its original insoluble form. Vat dyes are particularly light and washing resistant. In most tints, brilliant colours can be achieved. Vat dyes got their name from the vats that were used to reduce indigo plants through fermentation in mediaeval Europe.
Because vat dyes are insoluble in water, they can't be used for dyeing directly. They become soluble in an alkali and acquire affinity for cellulose fibres when reduced to a leuco form (colourless). Dyeing or printing can be done with a leuco form solution. The original insoluble dye is generated within the fibre structure during oxidation. This group of dyes includes indigo and indigosol O.
Key takeaways:
- Because vat dyes are insoluble in water, they can't be used for dyeing directly. They become soluble in an alkali and acquire affinity for cellulose fibres when reduced to a leuco form (colourless). This group of dyes includes indigo and indigosol O.
- A mordant, also known as a dye fixative, is a chemical that is used to set (or bind) dyes on fabrics by establishing a coordination complex with the dye and then attaching it to the cloth (or tissue). It can be used to colour fabrics or to make stains in cell or tissue preparations more intense.
4.12.1 The Chemistry of the Dyeing Process
Exhaustion in any dyeing process, whatever the chemical class of dye being used, heat must be supplied to the dye bath; energy is used in transferring dye molecules from the solution to the fiber as well as in swelling the fiber to render it more receptive. The technical term for this process is exhaustion. Levelness: An Important Quality
Evenness of dyeing, known as levelness is an important quality in the dyeing of all forms of natural and synthetic fibers. It may be attained by the control of dyeing conditions viz.
- By agitation to ensure proper contact between dye liquor and substance being dyed and by use of restraining agents to control rate of dyeing or strike. Solvent Dyeing Serious consideration has recently been given to the methods of dyeing in which water as the medium is replaced by solvents such as the chlorinated hydrocarbons used in dry cleaning. The technological advantages in solvent dyeing are: 1. Rapid wetting of textiles
2. Less swelling
3. Increased speed of dyeing per given amount of material
4. Savings in energy, as less heat is required to heat or evaporate per-chloro-ethylene. Thus, it eliminates the effluent (pollution) problems associated with the conventional methods of dyeing and finishing.
Machinery and Equipment: Modern dyeing machines are made from stainless steels. Steels containing up to 4% molybdenum are favoured to withstand the acid conditions that are common.
A dyeing machine consists essentially of a vessel to contain the dye liquor, provided with equipment for heating, cooling and circulating the liquor into and around the goods to be dyed or moving the goods through the dye liquor. The kind of machine employed depends on the nature of the goods to be dyed. Labor and energy costs are high in relation to total dyeing costs: the dyers aim is to shorten dyeing times to save steam and electrical power and to avoid spoilage of goods.
The conical-pan loose-stock machine is a widely used machine. Fibers are held in an inner truncated conical vessel while the hot dye liquor is mechanically pumped through. The fiber mass tends to become compressed in the upper narrow half of the cone, assisting efficient circulation. Leveling problems are less important as uniformity may be achieved by blending the dyed fibers prior to spinning.
The Hussong machine is the traditional apparatus. It has a long, square-ended tank as a dye bath into which a framework of poles carrying hanks can be lowered. The dye liquor is circulated by an impeller and moves through a perforated false bottom that also houses the open steam pipe for heating. In modern machines, circulation is improved at the points of contact between hank and pole. This leads to better leveling and elimination of irregularities caused by uneven cooling. In package-dyeing machines dye color may be pumped in rather two directions:
- Through the perforated central spindle and outward through the package or
2. By the reverse path into the outer layers of the package and out of the spindle. In either case levelness is important.
Some package-dyeing machines are capable of working under pressure at temperatures up to 130C.
The winch is the oldest piece of dyeing machine and takes its name from the slated roller that moves an endless rope of cloth or endless belt of cloth at full width through the dye liquor. Pressurized-winch machines have been developed in the U.S.
In an entirely new concept, the Gaston County jet machine circulates fabric in rope form through a pipe by means of a high-pressure jet of dye color. The jet machine is increasingly important in high-temperature dyeing of synthetic fibers, especially polyester fabrics. Another machine is the jig. It has a V-shaped trough holding the dye color and guide rollers to carry the cloth at full width between two external, powered rollers, the cloth is wound onto each roller alternately, that is, the cloth is first moved forward, then backward through the dye color until dyeing is complete. Modern machines, automatically controlled and programmed, can be built to work under pressure.
4.13.1 Synthesis of Methyl orange:
You will make methyl orange, an azo dye that forms gorgeous orange crystals and is utilised as an acid-base indicator, in this experiment. The anion is yellow, while the acid is red.
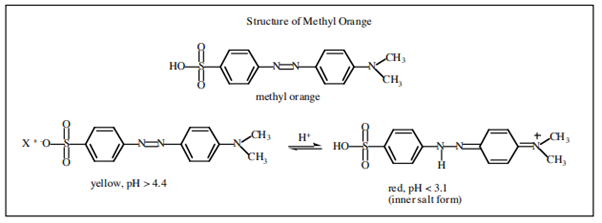
You will synthesize methyl orange from sulfanilic acid and N, N-dimethylaniline using a diazonium coupling reaction just like the one you saw in the previous experiment in the nitrous acid test for primary aromatic amines. The overall reaction is shown in Figure and the mechanism is shown in another figure. All of this chemistry is in your text in the chapter on amines. You need to understand it.
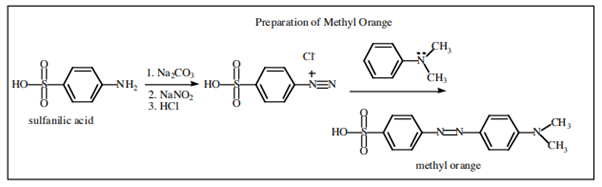
An acid-base reaction is the initial stage. We use sodium carbonate to dissolve the sulfanilic acid in the aqueous solution. The diazonium salt is then formed using the same technique as in Figure of the preceding experiment. When we add HCl, sodium nitrite forms a nitroso ion, which combines with the amine to form a nitroso ammonium adduct, which loses water under acidic circumstances after proton transfer. The diazonium salt is formed as a result of this process. At low temperatures, aromatic diazonium salts remain stable. The diazonium salt's terminal nitrogen is electron-deficient. It's vulnerable to excellent nucleophiles. The dimethylaniline is dissolved in acetic acid. The dimethylaniline acetate salt is formed as a result of this reaction. Due to the activating effect of the dimethylamine substituent, neutralise this in situ, and the dimethylaniline becomes a suitable nucleophile. Due to the bulky dimethylamine substituent's hindrance in the ortho position, the attack is in the para position.
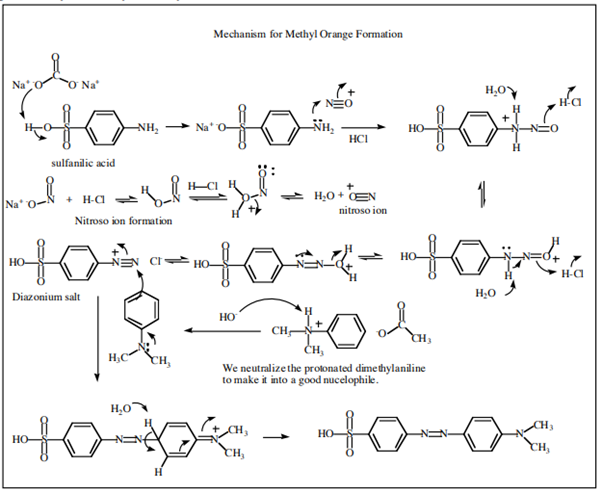
Physical constants
Compound | Mol. Wt(g/mol) | Density(g/mL) | b.p.  | m.p.  |
Sulfanilic acid | 173.19 | Solid | - | - |
N, N-Dimethylaniline | 121.18 | 0.956 | 193-194 | 1.5-25 |
Sodium carbonate (anhydrous) | 105.99 | Solid | - | 851 |
Sodium carbonate (monohydrate) | 124.01 | 2.250 | - | - |
Sodium nitrite | 69.00 | 2.168 | - | 271 |
Acetic acid | 60.05 | 1049 | 116-117 | 15-16 |
Methyl orange | 306 |
|
|
|
Procedure:
You must balance the quantities of solid reagent very carefully to the precision of 0.05 g or better in order to avoid any excess of reagent that could breakdown or cause decomposition and cause tar to contaminate our dye.
In this experiment, you'll have to figure out some of the reagent amounts on your own. Before continuing, confirm your results with the instructor after you've computed them.
In a 125 ml Erlenmeyer flask, dissolve 0.010 mole of sulfanilic acid (anhydride) in about 50 ml of a sodium carbonate solution containing 0.010 to 0.0125 moles of sodium carbonate. The stockroom prepares the solution, which is specified on the bottle by its strength, but you must determine the exact amount required. To speed up the dissolving, warm the mixture slightly. To make sure the solution is alkaline, take a drop and test it. If not, add a tiny amount of sodium carbonate solution (1-2 mL) and retest the pH. Then add 0.010 moles sodium nitrite and bring to a temperature of 25 °C (room temperature).
In a 400 mL beaker, put 40 g of ice and enough 6M or 12 M hydrochloric acid to make a total of 0.030 mol HCl.
In a fine stream, add the sulfanilate solution made above, stirring constantly. In the ice bath, keep this fluid cold at all times. Your diazonium salt is now inside, and if it gets too warm, it will decay. It will precipitate as a bluish-greenish solid since it is only partly soluble in aqueous solution.
In a 25 ml Erlenmeyer flask, make a solution of N, N-dimethylaniline (0.010 mol) in 0.010 mol acetic acid.
Slowly pour the dimethylaniline acetate solution into the diazonium salt suspension, stirring constantly. The result should be a drab, reddish-purple mass.
Now, slowly pour in about 30 mL of 1.0 M sodium hydroxide solution, stirring constantly. Adding a few mL of NaOH at a time. It should take 10 to 15 minutes to complete the addition. The true coupling reaction does not take place until the NaOH is added. The optimal pH for the reaction is around 7. Continue to add NaOH until the solution is basic (blue to litmus.)
Free dimethylaniline will separate out as an oily phase if the sodium hydroxide is applied too rapidly. As a result, an equivalent amount of diazonium salt remains unreacted. When the surplus salt is brought to room temperature, it decomposes into brown tar, contaminating the otherwise lovely crystalline orange colouring
A yellow-orange or golden tint should be visible at the end of the coupling reaction. From the reaction mixture, the product will now be recrystallized. Using your tripod and Bunsen burner, bring the reaction mixture to a boil. The solution should be transparent and everything should dissolve (though it will be highly colored). If the material does not dissolve completely after the solution has been brought to a boil, add more water as needed. The flask should then be allowed to cool slowly to room temperature to crystallise before being placed in an ice bath to get as cold as possible. When the solution is cooling, remember not to stir or shake it. Allow the crystals to develop in a flask that has not been disturbed. If they form slowly in a motionless flask, they will be more purer and larger.
Suction filter the crystals, then rinse them with 10 – 15 mL cool water and let them dry. Calculate the percent yield and submit it along with your Organic Yield Report Sheet in a vial.
Do not attempt to determine your methyl orange's melting point because it decomposes when heated. To view the lovely colour shift that occurs with our orange indicator dye, dissolve a small bit in 2-3 mL 95 percent ethanol and add 5 percent HCl a few drops at a time until you notice the colour shift. In your notepad, write down your findings.
4.13.2 The mechanism of diazo coupling:
The diazo-coupling reaction dates back to the 1850s (and a close association with Imperial College via the first professor of chemistry there, August von Hofmann) and its mechanism was much studied in the heyday of physical organic chemistry. Nick Greeves, purveyor of the excellent ChemTube3D site, contacted me about the transition state (I have commented previously on this aspect of aromatic electrophilic substitution). ChemTube3D recruits undergraduates to add new entries; Blue Jenkins is one such adding a section on dyes.
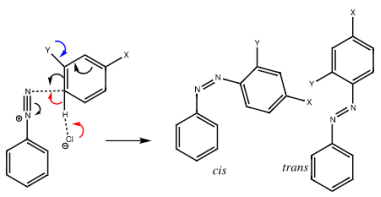
Either in the initial electrophilic attack (black arrows) or in the following proton removal, the mechanism can be rate limiting (red arrows using an intermolecular base such as chloride anion). The trans-diazo molecule is usually thought to be the product rather than the cis-diazo compound. In the crystal structure database, this dispersion is unmistakable (below, although some examples of cis are known, including azobenzene itself). Would the transition states reflect this distribution? The ChemTube3D team's initial efforts had only yielded a cis-transition state, so they asked me to investigate further.
ωB97XD/6-311G (d, p)/SCRF=water calculations using phenyl diazonium chloride (I do like my counter-ions) coupling to benzene resulted in location of both cis and trans transition states, the former being the lower by 1.0 kcal/mol in free energy (this might well be due to the dispersion stabilisation from π-π stacking). † The IRC for the cis is shown below.
You can see how well-coordinated the entire operation is. The Wheland intermediate, which is usually mentioned as part of the process of aromatic electrophilic substitution, is actually a hidden intermediate in the X=Y=H process. The reaction coordinate has a flat top, and the concealed Wheland is represented by the passage along this section. However, the reaction barrier is substantial, and only activated arenes (phenols, anilines, X, Y=OH, NH2) are observed to couple with diazonium cations. The substituent stabilises the concealed intermediate in these cases, and it undoubtedly emerges as a true intermediate.
Diazo-coupling of indoles was the subject of my thesis research. I might revisit that work to see if calculations can reproduce my discovery that proton removal from the Wheland intermediate is fast for unimpeded indoles, but slow with a few t-butyl hindrance groups.
The IRC for the formation of trans-diazobenzene.
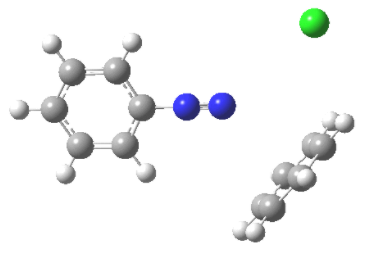
Such diazo compounds account for a sizable fraction of the 50 or so genuine molecules I've personally added to the 84 million or so that have been identified thus far.
When working with ions, there is one statistical challenge that covalent systems do not have: determining where the counter-ion should be geometrically placed. Before establishing the likely position of the worldwide lowest energy posture, one should stochastically examine reasonable locations.
4.14.1 Triphenylmethane dye:
Triphenylmethane dye refers to a range of synthetic organic dyes with molecular architectures inspired by the molecule triphenylmethane. They have low light and chemical bleach resistance and are mostly employed in copying papers, hectograph and printing inks, and textile applications where lightfastness is not a concern.
A practical procedure for the synthesis of fuchsine was invented in 1859, making triphenylmethane derivatives one of the oldest man-made colours. A number of other members of the class were found before their chemical makeup was fully understood. The most important of the series, crystal violet, was debuted in 1883.
The colour palette isn't exhaustive, but it contains reds, violets, blues, and greens. They're applied in a variety of ways, but the majority are from the basic class, which are absorbed from solution by silk or wool, but have no affinity for cotton until it's been treated with a mordant like tannin.
4.14.2 Malachite green:
Malachite green is an organic chemical used as a colour as well as a contentious antibiotic in aquaculture. Malachite green has been used as a dye for silk, leather, and paper for centuries. Despite its name, the dye is not made from the mineral malachite; the name is derived from the color's likeness.
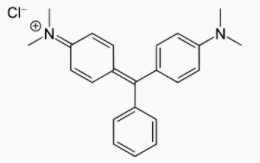
Structure
Structures and properties
Malachite green is classified in the dyestuff industry as a triarylmethane dye and also using in pigment industry. Formally, malachite green refers to the chloride salt [C6H5C(C6H4N(CH3)2)2]Cl, although the term malachite green is used loosely and often just refers to the colored cation. The oxalate salt is also marketed. The anions have no effect on the color. The intense green color of the cation results from a strong absorption band at 621 nm (extinction coefficient of 105 M−1 cm−1).
Malachite green is prepared by the condensation of benzaldehyde and dimethylaniline to give leuco malachite green (LMG):
C6H5CHO + 2 C6H5N(CH3)2 → C6H5CH(C6H4N(CH3)2)2 + H2O
Second, this colorless leuco compound, a relative of triphenylmethane, is oxidized to the cation that is MG:
C6H5CH(C6H4N(CH3)2)2 + HCl + 1⁄2 O2 → [C6H5C(C6H4N(CH3)2)2]Cl + H2O
A typical oxidizing agent is manganese dioxide.


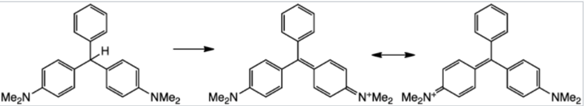
On the left is leuco-malachite Green (LMG) and on the right are the two equivalent resonance structures of the MG cation. The alcohol derivative of MG is derived from LMG by replacement of the unique C-H by C-OH
Hydrolysis of MG gives an alcohol:
[C6H5C(C6H4N(CH3)2)2]Cl + H2O → C6H5C(OH)(C6H4N(CH3)2)2 + HCl
This alcohol is significant because, unlike MG, it can pass across cell membranes. It is metabolised into LMG once within the cell. Only the cation MG has a rich hue, while the leuco and alcohol derivatives do not. Because only the cationic version has prolonged pi-delocalization, which allows the molecule to absorb visible light, this discrepancy develops.
Preparation:
The leuco form of malachite green was first prepared by Hermann Fischer in 1877 by condensing benzaldehyde and dimethylaniline in the molecular ratio 1:2 in the presence of sulfuric acid.
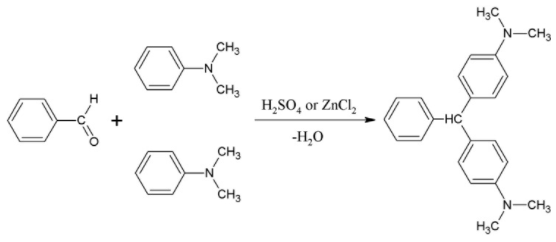
Uses
Malachite green is traditionally used as a dye. Kilotonnes of MG and related triarylmethane dyes are produced annually for this purpose.
MG is active against the oomycete Saprolegnia, which infects fish eggs in commercial aquaculture, MG has been used to treat Saprolegnia and is used as an antibacterial. It is a very popular treatment against Ichthyophthirius multifiliis in freshwater aquaria. The principal metabolite, LMG, is found in fish treated with malachite green, and this finding is the basis of controversy and government regulation. See also Antimicrobials in aquaculture.
MG has frequently been used to catch thieves and pilferers. The bait, usually money, is sprinkled with the anhydrous powder. Anyone handling the contaminated money will find that on upon washing the hands, a green stain on the skin that lasts for several days will result.
Niche uses
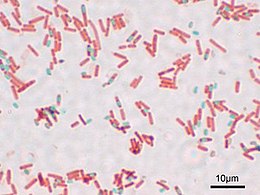
A preparation of Bacillus subtilis showing endospores stained with malachite green (vegetative cells-stained pink with safranin counterstain)
The bright hue of MG is used in a variety of specialty applications. It's a biological stain that's used to examine cell biology and tissue samples under a microscope. Basic fuchsin stains bacteria red or magenta, while malachite green is employed as a blue-green counterstain in the Gimenez staining procedure. Because malachite green can directly stain endospores within bacterial cells, it is also employed in endospore staining; however, a safranin counterstain is frequently utilised. Alexander's pollen stain includes malachite green. Malachite green can also be used as a saturable absorber in dye lasers or as a pH indicator with a pH range of 0.2 to 1.8. However, this is a somewhat uncommon application. In forensic science, leuco-malachite green (LMG) is utilised as a latent blood detection procedure. The reaction between LMG and hydrogen peroxide is catalysed by haemoglobin, which turns the colourless LMG into malachite green. As a result, the presence of blood is indicated by the emergence of a green colour.
4.14.3 Crystal violet:
A triarylmethane dye used as a histological stain and in Gram's technique of classifying bacteria, crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triarylmethane dye used as a histological stain and in Gram's method of classifying bacteria. Crystal violet was once used as a topical antiseptic because of its antibacterial, antifungal, and anthelmintic qualities. Although it is still classified by the World Health Organization, the dye's medical application has mostly been replaced by more sophisticated medications.
The name gentian violet was first applied to a combination of methyl pararosaniline dyes (methyl violet), although it is now frequently used interchangeably with crystal violet. It is not made from gentians or violets, and its name alludes to its colour, which is similar to the petals of certain gentian flowers.
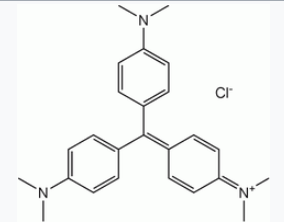
Structure
Production:
Crystal violet can be made in a number of different ways. Kern and Caro created the initial process, which required reacting dimethylaniline with phosgene to produce 4,4′-bis(dimethylamino)benzophenone (Michler's ketone) as an intermediate. In the presence of phosphorus oxychloride and hydrochloric acid, this was then reacted with further dimethylaniline.
The dye can also be prepared by the condensation of formaldehyde and dimethylaniline to give a leuco dye:
CH2O + 3 C6H5N(CH3)2 → CH(C6H4N(CH3)2)3 + H2O
Second, this colourless compound is oxidized to the coloured cationic form: (A typical oxidizing agent is manganese dioxide).
CH(C6H4N(CH3)2)3 + HCl + 1⁄2 O2 → [C(C6H4N(CH3)2)3]Cl + H2O
Dye colour:
When dissolved in water, the dye has a blue-violet colour with an absorbance maximum at 590 nm and an extinction coefficient of 87,000 M−1 cm−1. The colour of the dye depends on the acidity of the solution. At a pH of +1.0, the dye is green with absorption maxima at 420 nm and 620 nm, while in a strongly acidic solution (pH −1.0), the dye is yellow with an absorption. The maximum wavelength is 420 nm.
The varied colours are caused by the dye molecule's distinct charged states. The yellow colour corresponds to a form of the dye in which all three nitrogen atoms have a positive charge, two of which are protonated, whereas the green colour relates to a version of the dye in which two nitrogen atoms are positively charged. Both extra protons are lost to the solution at neutral pH, leaving only one nitrogen atom positively charged. The pKa values for the loss of the two protons are 1.15 and 1.8, respectively. Nucleophilic hydroxyl ions attack the electrophilic core carbon in alkaline solutions, resulting in the colourless triphenylmethanol or carbinol form of the dye. Under very acidic conditions, some triphenylmethanol is generated when the positive charges on the nitrogen atoms increase the electrophilic character of the central carbon, allowing water molecules to attack it nucleophilically. The golden colour fades slightly as a result of this impact.

Applications:
1. Nonmedical
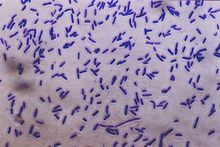
Bacteria stained with crystal violet
Crystal violet is used to dye paper and is a component of navy blue and black inks for printing, ball-point pens, and inkjet printers, among other things. It's also used to colour fertilisers, antifreezes, detergents, and leather, among other things. The dye is also employed as a histology stain, especially in Gram staining, which is used to characterise bacteria. Crystal violet can be used as a nontoxic DNA stain instead of fluorescent intercalating dyes like ethidium bromide when performing DNA gel electrophoresis. It can be included into the agarose gel or added after the electrophoresis process is complete when used in this way. It can detect as little as 16 ng of DNA when used at a concentration of 0.001% and allowed to stain a gel after electrophoresis for 30 minutes. Sensitivity can be increased to 8 ng of DNA by using a methyl orange counterstain and a more sophisticated staining procedure. It is not essential to utilise ultraviolet light when using crystal violet as an alternative to fluorescent stains; this has made crystal violet useful as a way to avoid UV-induced DNA degradation while performing DNA cloning in vitroCrystal violet can be used to label the nuclei of adhering cells in biomedical research. Crystal violet is used as an intercalating dye in this application, allowing for the quantification of DNA that is proportionate to the number of cells Crystal violet was used to produce fingerprints in forensics. In the production of light microscopy sections, crystal violet is frequently utilised as a tissue stain. Because most cells are colourless, solutions containing crystal violet and formalin are frequently employed in laboratories to simultaneously fix and stain cells grown in tissue culture to preserve them and make them easily visible. It's also occasionally used as a cheap technique to label research mice with identification markings; because many lab mouse strains are albino, the purple colour lingers on their coats for several weeks. Gentian violet is widely used to identify the place for piercings, including surface piercings, in body piercing.
2. Medical
Antibacterial, antifungal, antihelminthic, antitrypanosomal, antiangiogenic, anticancer activities are all found in gentian violet. It is also known as "pyoctanin" and is utilised medically for these qualities, particularly in dentistry (or "pyoctanine"). It's frequently used for: • Marking the skin in preparation for surgery and allergy tests; Treating Candida albicans and related fungal illnesses such thrush, yeast infections, and tinea (ringworm, athlete's foot, and jock itch); Treating impetigo; it was first used before antibiotics, but it's still effective for people who are allergic to penicillin. Burn wounds, inflammation of the umbilical cord stump (omphalitis) in the new born period, oral candidiasis in HIV-infected individuals, and mouth ulcers in children with measles are all treated with gentian violet in resource-limited settings.
4.15.1 Molecular structure of fluorescein (A) and colored phenolphthalein (B).
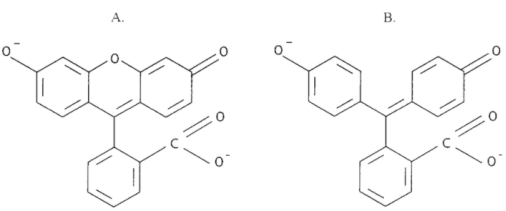
4.15.2 Phenolphthalein:
Phenolphthalein, (C20H14O4), an organic compound of the phthalein family that is widely employed as an acid-base indicator. As an indicator of a solution’s pH, phenolphthalein is colourless below pH 8.5 and attains a pink to deep red hue above pH 9.0.

Phenolphthalein, which is ordinarily colourless, turns a bright red colour when it reaches pH 10.0.
Phenolphthalein is a strong laxative that takes effect in 6–8 hours and might linger for 3–4 days. It's possible that you'll get side effects including kidney irritation or a rash on your skin. Phenolphthalein was commonly used in over-the-counter laxatives until it was prohibited in 1999 by the US Food and Drug Administration because animal research suggested it could cause cancer in humans.
Phenolphthalein was discovered in 1871 by German chemist Adolf von Baeyer, who made it by fusing phenol and phthalic anhydride in the presence of sulfuric acid or zinc chloride, a method that is still used today.
4.15.3 Phthalein:
Phthalein is a colourless, odourless substance Condensation of phthalic anhydrid with phenols produces a group of coloring-matters. When administered internally, several phthaleins have a purgative effect. They play an important role in colour chemistry, and several of its derivatives are useful dyestuffs. Phenolphthalein and phthalic anhydrid are two examples of phthalic anhydrid.
4.15.4 Fluorescein:
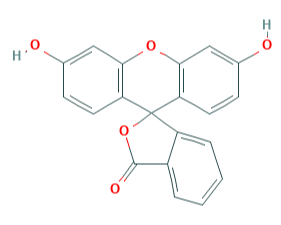
Fluorescein is a dye used in angiography or angioscopy of the iris and retina.
Fluorescein, also called Resorcinolphthalein, organic compound of molecular formula C20H12O5 that has wide use as a synthetic colouring agent. It is prepared by heating phthalic anhydride and resorcinol over a zinc catalyst, and it crystallizes as a deep red powder with a melting point in the range of 314° to 316° C (597° to 601° F). Fluorescein was named for the intense green fluorescence it imparts to alkaline solutions—a colour visible even at dilutions of 1:50,000,000. It is used as a dye to colour liquids in analytic instruments, in cosmetics, and as a water tracer or marker. Halogenated derivatives made from fluorescein also include eosin and erythrosin.

Fluorescein powder
Key takeaways:
When compared to phenolphthalein (= 0.21 10 4 M 1 cm1), fluorescein has a molar absorption coefficient that is twice as high (= 0.46 10 4 M 1 cm1). • Fluorescein and eosin have rigid structures and are intensely fluorescent, whereas phenolphthalein is non-rigid and non-fluorescent due to the existence of the O-bridge, which makes the fluorescein molecule rigid. Fluorescence spectroscopy is a spectroscopic method for detecting the concentration of an analyte in a sample and analysing its fluorescence properties.
References:
1. Talwar, G.P. & Srivastava, M. Textbook of Biochemistry and Human Biology, 3rd Ed. PHI Learning.
2. Berg, J.M., Tymoczko, J.L. & Stryer, L. Biochemistry, W.H. Freeman, 2002.
3. Murray, R.K., Granner, D.K., Mayes, P.A. And Rodwell, V.W. (2009) Harper’s Illustrated Biochemistry. XXVIII edition. Lange Medical Books/ McGraw-Hill.
4. Berg, J.M., Tymoczko, J.L. And Stryer, L. (2006) Biochemistry, 6th Edition. W.H. Freeman and Co. (2002).
5. Wilson, K. & Walker, J. Practical Biochemistry. Cambridge University Press (2009).