Module 1
Fossil Fuel
- Sources of energy:
Introduction: Fuel is a substance that is burned to provide nuclear energy, heat, and power. These are the sources of energy and power. Materials like coal, wood, gas, or oil can provide while burning. Some sources of energy are mentioned below:
Fossil energy sources are sources that include oil, coal, and natural gas that is formed when prehistoric plants and animals died and were gradually buried by layers of rock. Over millions of years, different types of fossil fuels formed depending on what combination of organic matter was present, how long it was buried, and under what pressure and temperature conditions existed there as the time passed. These are conventional and traditional energy resources.
In today’s scenario, fossil fuel industries are responsible for excavations and extraction of these fuels by using several methods of drilling, mining, and burning these fuels to produce heat and power.
The potential energy of water is utilized to move the turbines, which in turn run the electric generators. It is the cleanest method of producing electric power.
C components of the hydroelectric power plant:
- Dam:
- The dam is the most important component of the hydroelectric power plant. The dam is built on a large river that has an abundant quantity of water throughout the year.
- It should be built at a location where the height of the river is sufficient to get the maximum possible potential energy from water.
Water Reservoir:
The water reservoir is the place behind the dam where water is stored. The water in the reservoir is located higher than the rest of the dam structure The height of water in the reservoir decides how much potential energy the water possesses. The higher the height of the water, the more its potential energy. The high position of water in the reservoir also enables it to move downwards effortlessly.
Intake or Control Gates:
- These are the gates built on the inside of the dam. The water from the reservoir is released and controlled through these gates. These are called inlet gates because water enters the power generation unit through these gates.
- When the control gates are opened the water flows due to gravity through the penstock and towards the turbines. The water flowing through the gates possesses potential as well as kinetic energy.
Water Turbines:
- Water flowing from the penstock is allowed to enter the power generation unit, which houses the turbine and the generator.
- When water falls on the blades of the turbine the kinetic and potential energy of water are converted into the rotational motion of the blades of the turbine. The rotating blades cause the shaft of the turbine to also rotate.
- The turbine shaft is enclosed inside the generator. In most hydroelectric power plants there is more than one power generation unit. There are various types of water turbines such as the Kaplan turbine, Francis turbine, Pelton wheels, etc.
Generators:
- It is in the generator where the electricity is produced. The shaft of the water turbine rotates in the generator, which produces an alternating current in the coils of the generator.
- It is the rotation of the shaft inside the generator that produces a magnetic field that is converted into electricity.
Working of hydroelectric power plant
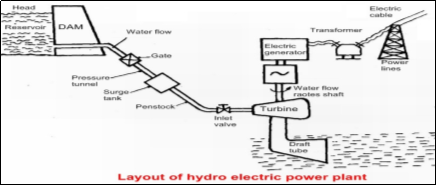
Fig. 1: Working Layout of a hydroelectric power plant
- The dam stores lots of water behind it in the reservoir. Near the bottom of the dam wall, there is the water intake. Gravity causes it to fall through the penstock inside the dam.
- At the end of the penstock, there is a turbine propeller, which is turned by the moving water. The shaft from the turbine goes up into the generator, which produces the power.
- Power lines are connected to the generator that carries electricity to your home and mine. The water continues past the propeller through the tailrace into the river past the dam.
- Solar energy is the energy that is in sunlight. It has been used for thousands of years in many different ways by people all over the world.
- As well as its traditional human uses in heating, cooking, and drying, it is used today to make electricity where other power supplies are absent, such as in remote places and space.
- It is becoming cheaper to make electricity from solar energy and in many situations, it is now competitive with energy from coal or oil.
- The sun is producing solar energy through nuclear fusion. In this reaction, two atoms, deuterium (heavy hydrogen) combine to form one atom of helium, and during the process, it releases a large amount of energy that reaches the earth's surface through electromagnetic radiation.
- The sun sends out the energy in the form of radiation at a rate of 3.7 x 1020 MW. However, the energy reached to the earth is about 1.85 x 1011 MW. This energy available is several times more than all the energy produced and consumed in the world today.
Solar collectors:
It is a device for collecting or absorbing the solar radiation on a surface called an absorber. Then, the Transfer of radiant energy to fluid like water or air in contact with the collector takes place. The surface of the collector is designed for high absorption and low emissions.
Power generation from solar energy
- Solar Thermal Power Plants
- Solar PV Cell Power Plants
Construction and working of solar thermal power plant
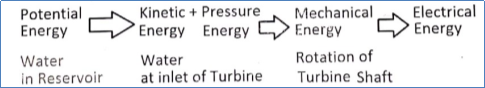
- It consists of an array of concentrating solar collectors, steam generator or Heat Exchanger, steam turbine, condenser, and generator as shown in the figure. The generator is attached to the steam turbine.
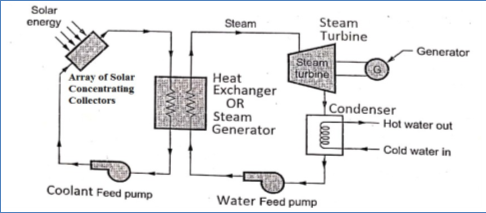
Fig.2: Working of solar power plant
- Concentrating solar collectors are used to produce high-pressure steam which is then used in the conventional steam power plants.
- Solar collectors capture and concentrate sunlight to heat the liquid inside the collector, which then heats water in the heat exchanger to create steam. The steam is piped to an onsite turbine-generator to produce electricity.
- Wind can be used as a power source. Wind power is the use of airflow through wind turbines to mechanically power generators for electricity.
- Wind power, as an alternative to burning fossil fuels, is plentiful, renewable, widely distributed, clean, produces no greenhouse gas emissions during operation, consumes no water, and uses little land.
- The net effects on the environment are far less problematic than those of non-renewable power sources.
- The wind power can be generated where the wind velocities are more than 8 kmph.
- Such winds are available along the sea coast and at high altitudes in the hilly region.
- The wind power is clean and non-polluting
- It has a low maintenance cost and low power generation cost of about Rs. 2.25/kWh.
- It needs a high capital cost of about 3.5crores/MW.
Construction and Working of wind power plant:
- It consists of the rotor blade, gearbox, generator, power cables, tower, and transformer. The blade is attached or connected to the nacelle.
- The gearbox is present inside the assembly and the generator is connected to it. Through the generator, cables are connected and supplied to directly the power supply system.
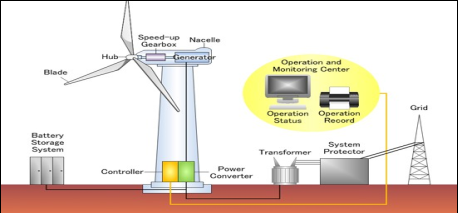
Fig.3: Working of wind energy power plant

- Wind (moving air that contains kinetic energy) blows toward the turbine's rotor blades. The rotors spin around, capturing some of the kinetic energy from the wind, and turning the central driveshaft that supports them.
- Although the outer edges of the rotor blades move very fast, the central axle (drive shaft) they're connected to the turbine turns quite slowly.
- The gearbox converts the low-speed rotation of the drive shaft into high-speed rotation fast enough to drive the generator. The generator, immediately behind the gearbox, takes the kinetic energy from the driveshaft and converts it into electrical energy.
- A nuclear power plant or nuclear power station is a thermal power station in which the heat source is a nuclear reactor. As is typical in all conventional thermal power stations the heat is used to generate steam which drives a steam turbine connected to an electric generator that produces electricity.
- Nuclear power is the large amount of energy that is released in the nuclear fusion reaction. It is a process in which the nucleus of an atom splits into smaller parts (lighter nuclei) and releases a very large amount of energy even by the energetic standards of radioactive decay. The difference in mass between the products and reactants is manifested as the release of large amounts of energy.
C omponent of a nuclear power plant
The main components of the nuclear power plant are:
- Nuclear reactor.
- Steam generator (Heat Exchanger)
- Steam turbine.
- Condenser.
- Electric generator
Nuclear reactor:
- In a nuclear reactor, energy is produced by the fission reaction of nuclear fuel. The nuclear fuels used in a nuclear reactor are unstable radioactive materials such as Thorium (Th 232), Uranium (U 235), or Plutonium (Pu 239)
- In nuclear fission, the slow-moving neutrons are bombarded on the atoms of nuclear fuel. The heavy nucleus of nuclear fuel like uranium is split into two or lighter nuclei having a combined mass less than the parent nucleus. The reduced mass is converted into enormous energy.
- This energy released is taken away by the primary coolant. The primary coolants may be a gas like carbon dioxide, helium, or liquid metals like sodium, potassium, and their alloys.
- There are three main components of a nuclear reactor are:
(i) moderator;
(ii) (ii) control rods; and
(iii) (iii) external shield.
(i) Moderator:
- The fission of nuclear fuel produces fast-moving neutrons. These neutrons are slowed down to the right speed by moderators to increase the effectiveness of the fission of nuclear fuel.
(ii)Control rods:
- The rate of energy released by chain reaction due to the fission of nuclear fuel is very high. If the rate of heat energy is not controlled properly, it may melt the entire core and the surrounding structures. The rate of heat energy released is controlled by adjustable rods called control rods, made of a material like cadmium or boron. The control rods regulate the energy release by absorbing neutrons. Note that the moderator reduces the speed of neutrons without absorbing them, while the control rods regulate the energy release by absorbing neutrons.
(iii)External shield:
An external shield is provided for the physical safety of persons operating the nuclear reactor, from harmful effects of radiation.
(iv)Heat Exchanger:
The heat absorbed by the primary coolant in the nuclear reactor is transferred to water in the heat exchanger. The water is converted into high-pressure steam. Therefore the heat exchanger acts as a boiler of a thermal(steam) power plant.
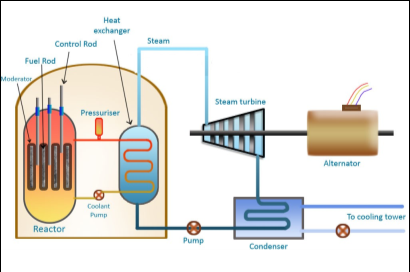
Fig. 4: Working layout of a nuclear power plant
Working of plant:
- When the uranium atom splits, it releases energy and two or more neutrons from its nucleus. These neutrons can then hit the nuclei of other uranium atoms causing them to fission.
- The sequence of one fission triggering others, and those triggering still more, is called a chain reaction when the atoms split, they release energy in the form of heat.
- The heat is transferred from the reactor core to the water flowing past. That water becomes high-pressure steam which turns the turbine into an electric generator.
- Biomass is an organic matter produced by plants both grown on land and grown on the water their derivatives and animal manure. The energy obtained from biomass is called biomass energy and these fuels are extracted from biomass is biofuel.
- Coal, petroleum oil, and natural gas do not come in the category of 'biomass', because they are produced from dead, buried biomass under pressure and temperature for millions of years. Biomass can also be considered a form of solar energy as the latter is used indirectly to grow these plants by photosynthesis.
Resources of biofuel:
- Concentrated wastes
(i) Municipal solid
(ii) Sewage wood products
(iii) Industrial waste
(iv) Manure at large lots.
- Dispersed waste residue:
(i) Crop residue
(ii) Logging residue
(iii) Disposed manure.
- Harvested biomass:
(i) Standing biomass
(ii) Biomass energy plantations.
Global warming is a phenomenon of climate change characterized by a general increase in average temperatures of the Earth, which modifies the weather balances and ecosystems for a long time. It is directly linked to the increase of greenhouse gases in our atmosphere, worsening the greenhouse effect.
The greenhouse effect is a natural phenomenon. However, the increase in greenhouse gases is linked to human activities. It is thus no surprise that the world's leading climate scientists believe that human activities are very likely the main cause of global warming.
Ozone depletion, gradual thinning of Earth’s ozone layer in the upper atmosphere caused by the release of chemical compounds containing gaseous chlorine or bromine from industry and other human activities. The thinning is most pronounced in the polar regions, especially over Antarctica. Ozone depletion is a major environmental problem because it increases the amount of ultraviolet (UV) radiation that reaches Earth’s surface, which increases the rate of skin cancer, eye cataracts, and genetic and immune system damage.
- It is a branch of science which deals with the study of energy transfer and its effects on properties of systems and surroundings. OR
- Thermodynamics is a science which deals with the relations among heat, work, and properties of the system which are in equilibrium. It describes state and changes in the state of physical systems.
- Thermodynamic System: A quantity of matter or fixed mass in a region in space chosen for the study of heat and work transfer is known as a thermodynamic system.
- Surroundings: Mass or region outside the system is called the surrounding. Everything outside the system can be called the surrounding.
- Boundary: It is the real or imaginary surface that separates the system from the surroundings. The boundary can be fixed or movable.
 Universe: A system & its surroundings together are called the universe.
Universe: A system & its surroundings together are called the universe.
Fig.5: Surroundings, boundary, and System
It is the condition of the body or object when all the properties have fixed values.
At a given state, all the properties of a system have fixed values. If the value of even one property changes, the state will change to a different one i.e. when any of the property changes, then the state of the object also changes.
Work is also a transient form of energy which is observed when it crosses the boundaries of the system without transfer of mass.
Heat is the form of energy that is transferred, without transfer of mass, from one body to another body from higher temperature to lower temp. It is denoted by Q.
Sign convention – Heat transfer to the system from the surrounding is considered +Ve & vice versa.
Statement -: ‘It states that if two bodies (or systems) are in thermal equilibrium with a third body (system) separately, then those two bodies are also in thermal equilibrium with each other. i.e. all three systems are in thermal equilibrium with each other.’
Observation-: When a body is brought into contact with another body that is at a different temperature, heat is transferred from the body at a higher temperature to the one at a lower temperature until both bodies attain the same temperature (thermal equilibrium).
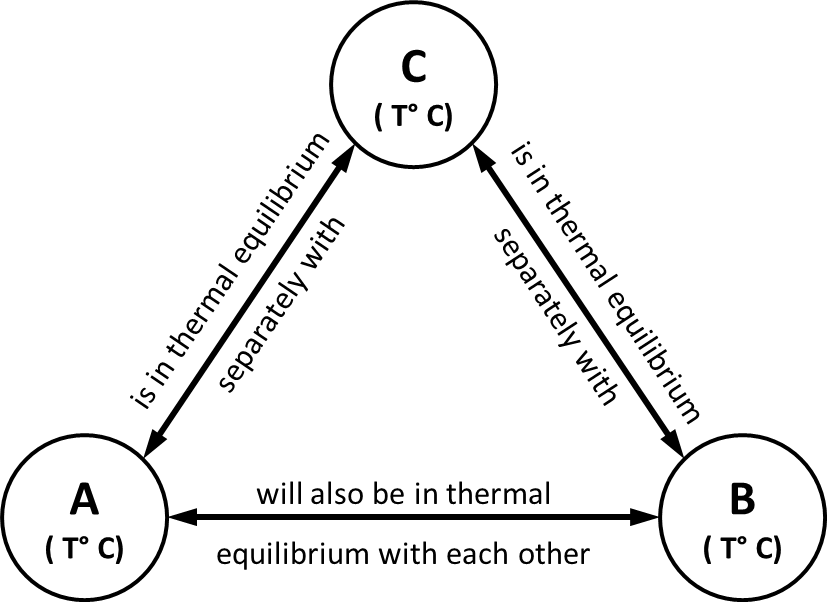
Fig. 6: Zeroth law of thermodynamics
Consider that there are three bodies A, B, C respectively having temperature T °C.
Let body A is in thermal equilibrium with body C as both have the same temperature T °C. Let body B is in thermal equilibrium with body C as both have the same temperature T °C. Now, since both bodies A & B are having a temperature of T °C as that of the temperature of body C, then Both A and B are also in thermal equilibrium with each other.
When a closed system undergoes a thermodynamic cycle then the net heat supplied to the system from the surroundings is equal to the network done by the system on its surroundings. OR
When a closed system executes a cyclic process, the algebraic sum of work transfers is proportional to the algebraic sum of heat transfer. W CYCLE QCYCLE
Energy can neither be created nor destroyed; but, it can be converted from one form to another form. OR
All the forms of energy are equivalent as well as convertible. Both heat and work are mutually convertible. OR
No machine can produce energy without the corresponding expenditure of energy, i.e., it is impossible to construct a perpetual motion machine of the first kind (PMM-1).
PMM-1 is a machine or device which violates or disagrees with the first law of thermodynamics.
Kelvin-Plank statement
It is impossible to construct a heat engine operating in a cyclic process, whose sole function is to transfer the entire heat received from a single heat reservoir & its conversion into an equal amount of work.
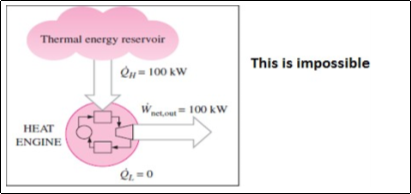
Fig. 7: Heat engine violating Kelvin-plank statement
Clausius statement:
It is impossible to construct a device (heat pump or refrigerator) operating in a cyclic process, whose sole function is to transfer the heat from a low-temperature heat reservoir to a higher temperature heat reservoir without any supply of input external work to the device.
The third law of thermodynamics states that a system's entropy approaches a constant value as the temperature approaches absolute zero. Except for non-crystalline solids (glasses), the entropy of a system at absolute zero is typically close to zero.
One of the thermodynamic properties of a system is its internal energy, E, which is the sum of the kinetic and potential energies of the particles that form the system. The internal energy of a system can be understood by examining the simplest possible system: an ideal gas. Because the particles in an ideal gas do not interact, this system has no potential energy. The internal energy of an ideal gas is, therefore, the sum of the kinetic energies of the particles in the gas.
Enthalpy:
- H= U + PV
- H is called specific enthalpy & U represent the specific internal energy
- H is an extensive property. The combination of (u + PV) plays an important role in analyzing the problems in the case of open systems where matter crosses the boundary of the system.
Entropy:
Entropy, the measure of a system's thermal energy per unit temperature that is unavailable for doing useful work. Because work is obtained from ordered molecular motion, the amount of entropy is also a measure of the molecular disorder, or randomness, of a system.
Steam is the gaseous phase of water. It utilizes heat during the process and carries large quantities of heat later. Steam is generated in boilers at constant pressure. Generally, steam may be obtained starting from ice or straight away from the water by adding heat to it.
If the wet steam is heated at constant pressure, its dryness fraction changes from x. During the process, the temperature remains constant (Isothermal) till it becomes dry saturated. It may be represented by a horizontal line AB on the p-v diagram.
Hence Isothermal and constant pressure Processes are identical. The adiabatic expansion of wet steam follows the curve CD. AB. The law for such expansion is approximately pV1.3 = constant. Once the steam becomes saturated, further heating will not be isothermal. Hence as soon as the steam becomes superheated it behaves like a perfect gas and will follow hyperbolic law (PV = constant) at a constant temperature. Hence isothermal and hyperbolic processes are identical for superheated steam.
References:
- J K Gupta and R S khurmi
- Various presentations from google.
- Notes developed by Shubham Tyagi
- Notes provided by the firm.