Unit-1
Electrochemistry and Energy Storage Systems
The branch which deals with the movement of energy from one form to the other and the relationship that exists between heat and temperature with energy and the work done is called as thermodynamics. Thermodynamics is a branch of Science that deals with the different forms of energy and work done in a system. Thermodynamics is only confined to Large scale response of a system which we are observed and measured in experiments
Energy:
It referred to the energy content that is present within the system. The energy represents the overall energy contained in the system and may include many forms of energy such as potential energy, kinetic energy etc. In a chemical reaction, we know the energy transformations and basic thermodynamics gives us information regarding energy change associated with the particles present in a system.
Factors affecting the internal energy
The internal energy of a system may change when:
- Heat passes into or out of the system,
- Work is done on or by the system or matter enters or leaves the system
Work:
Work done by a system is defined as the amount of energy exchanged between a system and its surroundings. Work is completely determined by external factors such as an external force, pressure or volume or change in temperature etc.
Heat:
Heat in thermodynamics is defined as the kinetic energy of the molecules of the substance or material. Heat and thermodynamics together form the basics which help process designers and engineers to optimize their processes and harness the energy associated with chemical reactions economically.
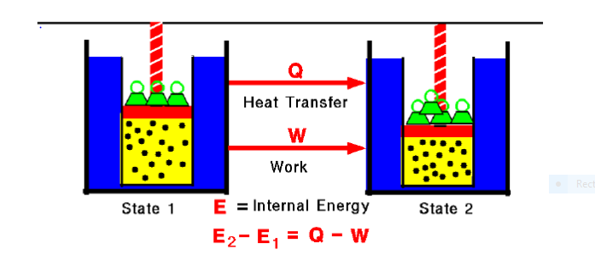
Fig. 1:
Any thermodynamic system in an equilibrium state possesses a state variable called the internal energy (E). Between any two equilibrium states, the change in internal energy is equal to the difference of the heat transfer into the system and work done by the system.
- Enthalpy:
Enthalpy a process or activity takes place at constant pressure, the heat released or absorbed is equal to the Enthalpy change. Enthalpy is occasionally called or known as “heat content”, “enthalpy” is derived from the Greek word which means “warming”. Enthalpy(H) is the sum of the internal energy(U) and the result of pressure(P) and volume(V).
Enthalpy H is written as,
H = U + PVm
Where, H = is the Enthalpy of the system
U = is the Internal energy of the system and is entirely dependent on the state functions T, p and U.
Enthalpy can also be written as
ΔH=ΔU+ΔPV.
P = is the Pressure of the system
V = Volume of the system Enthalpy is not directly measured, but the change in enthalpy (ΔH) is measured, which is the heat lost or added by the system,
Entropy:
It was Introduced by the German Physicist Rudolf Clausius in 1850, and is a highlight of 19th century Physics. The word “entropy” is derived from the Greek word, which means “turning”. It was derived to provide a quantitative measure for the spontaneous changes and Clausius introduced the concept of entropy as a precise way of expressing the Second Law of Thermodynamics, Clausius form of the second law states that spontaneous change for an irreversible process in an isolated system
Is a measure of randomness or irregularity or disorder of the system?
The more or the randomness, higher is the entropy.
Solid state has the lowest or least entropy, the gaseous state has the highest entropy and the liquid state has the entropy that lies between the two.
Entropy is a state function. The changes in its value during any process, is called the entropy change.
ΔS = S2 -S1 = ∑S products – ∑S reactants
1) When a system absorbs heat, the molecules begin to move faster because the kinetic energy increases. Therefore, disorder increases. Greater the heat absorbed, greater will be the disorder.
2) For equal amount of heat absorbed at low temperature, the disorder will be more than at high temperature. This proves that entropy change is inversely proportional to temperature.
ΔS = eve / T
Entropy change during a process is defined as the quantity of heat (q) absorbed isothermally and reversibly divided by the absolute Temperature (T) at which the heat is absorbed
- Free Energy
Gibbs Free Energy is a measure of the potential for reversible or maximum work that may be done by a system at constant temperature and pressure. It is a thermodynamic function that was discovered in 1876 by Josiah Willard Gibbs to assume if a process will occur spontaneously at constant temperature and pressure. Gibbs free energy G is defined as
G = H - TS
Where H, T, and S are the Enthalpy, temperature, and entropy. The SI unit for Gibbs energy is the kilojoule.
Estimations of entropy and free energies
Gibbs free energy combines both the enthalpy and entropy into a single value.
Gibbs free energy is the energy t with a that associates itself with a chemical reaction. It equals the enthalpy minus the temperature of the product and the entropy of the system.
G=H-TS
At constant temperature
ΔG = ΔH – TΔS
ΔG predicts the direction of a chemical reaction. If ΔG value is negative, then the corresponding reaction is spontaneous. If ΔG value is positive then reaction is non-spontaneous.
ΔGº=ΔHº-TΔSº
Where
ΔGº = Gibbs free energy (J or KJ)
ΔHº=enthalpy
T=Temperature
ΔSº=Entropy
Gibbs free energy is the energy that is available to do quality work.
A reaction will spontaneously occur if ΔG<0 (exergonic reaction)
A reaction will not spontaneously occur if ΔG>0 (endergonic reaction).
If ΔG value is less than zero, there is a thermodynamic force for the reaction or it drives the process in the forward direction.
When ΔG is positive, then reactants are favoured, when ΔG=0 system is at equilibrium.
Electromotive force, also known as cell potential or, as it is often written, e.m.f., is described as that source of energy which enables electrons movement around an electric circuit.
For any object to move from rest, there has to be some energy change. To ensure electrons movement round an electrical circuit, they should receive energy from a source of e.m.f. Which usually is a battery or a generator.
For every coulomb of electricity to move completely around an electrical circuit, a certain amount of electrical energy is needed, which depends on the particular circuit. The e.m.f. Is expressed in volts and is numerically the number of joules of energy given by the source of e.m.f. To each coulomb to enable movement around the circuit. The symbol for volt is the capital letter V.
Thus
Joules colombs=volts.
It follows that:
Joules=volts × coulombs = volts × ampers × seconds.
A 12-volt (12-V) battery is able to give 12 joules (12 J) of energy to each coulomb to enable movement around an electrical circuit.
The symbol for e.m.f. Is the capital letter E.
Example:
Calculate the energy supplied by a 12v battery when a current of 4 A flows for 10 minutes.
Energy supplied = Volts x amperes x seconds
= 12 x 4 x (10 x 60) Joules
= 28,800 J
Cell Potential
(1) “The difference in potentials of the two half – cells of a cell known as electromotive force (emf) of the cell or cell potential.”
The potential difference of the two half – cells of a cell arise because of the flow of electrons from anode to cathode and flow of current from cathode to anode.
(2) The emf of the cell or cell potential can be calculated from the values of electrode potentials of two half – cells constituting the cell. The following three methods are in use
(i) When oxidation potential of anode and reduction potential of cathode are taken into consideration
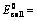 Oxidation potential of anode + Reduction potential of cathode
Oxidation potential of anode + Reduction potential of cathode 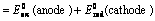
(ii) When reduction potentials of both electrodes are taken into consideration
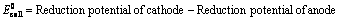
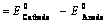
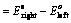
(iii) When oxidation potentials of both electrodes are taken into consideration
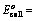 Oxidation potential of anode – Oxidation potential of cathode
Oxidation potential of anode – Oxidation potential of cathode 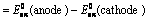
Any change in the Gibbs free energy G directly correspond to changes in free energy for processes at constant temperature and pressure, change is the maximum non-expansion work obtainable under these conditions in a closed system; ΔG is negative for spontaneous process, positive for nonspontaneous process, and zero for processes at equilibrium.
It takes into consideration the values of the standard electrode potentials, temperature, activity and the reaction quotient for the calculation of cell potential. For any cell reaction, that occurs Gibbs free energy can be related to standard electrode potential as:
ΔG =-nFE
Where, n = number of electrons transferred in the reaction, ΔG= Gibbs free energy, E= cell potential F = Faradays constant (96,500 C/mol) and. Under standard conditions, the above equation can be written as,
ΔGo =-nFEo
According to the theory of thermodynamics, Gibbs free energy under general conditions can be related to Gibbs free energy under standard condition and the reaction quotient as:
ΔG=ΔGo + RT lnQ
Where, Q= reaction quotient, R= universal gas constant and T= temperature in Kelvin. Incorporating the value of ΔGo and ΔG, from the first two equations, we get the equation:
-nFE = -nFE0 + RT lnQ
E = E0 – (RT/nF) lnQ
By conversion of Natural log to log10, the above equation is called as the Nernst equation. Here, it shows the relation of the reaction quotient and the cell potential. Special cases of Nernst equation:
E = Eo − (2.303RT/nF) log10Q
At standard temperature, T= 298K:
E = Eo − (0.0592V/n) log10Q
At standard temperature T = 298 K, the 2.303RTF, term equals 0.0592 V.
Under Equilibrium Condition
As the redox reaction in the cell progresses, the concentration of reactants decreases while the concentration of products increases. This process goes on until equilibrium is achieved. At equilibrium, ΔG = 0. Hence, cell potential, E = 0. Thus, the Nernst equation can be modified to:
E0 – (2.303RT/nF) log10Keq = 0
E0 = (2.303RT/nF) log10Keq
Where, Keq = equilibrium constant and F= faradays constant. Therefore, the above equation gives us a relation between standard electrode potential of the cell where the reaction takes place and the equilibrium constant.
NUMERICAL ON NERNST EQUATION – EXAMPLE
Q. Find the cell potential of a galvanic cell based on the following reduction half-reactions at 25 C.
Cd2++2e−→Cd−Eo=−0.403V
Pb2++2e−→Pb−Eo=−0.126V where, [Cd2+] =0.02M, [Pb2+] =0.2M
Solution:
The first step is to determine the cell reaction and total cell potential.
In order for the cell to be galvanic, reactions need to be spontaneous i.e., EOcell>0.
Since Cadmium is having lesser reduction potential amongst the two, Cadmium must undergo oxidation. Hence reactions involved will be:
Cd→Cd2++2e−, Pb2++2e−→Pb
Hence, overall reaction will be:
Pb2++Cd→Pb+Cd2+
EOcello=+0.403−0.126=0.277V
Now, from Nernst Equation we have,
Ecell=EOcell−0.0591/n logQ at 25oC
Here, we can write Nernst Equation as,
Ecell=0.277−0.059/2 log(0.20/.02)
i.e. Ecell=0.300V
Reference electrodes:
Calomel electrode
The Calomel electrodes is used in voltmeter and also pH meters, it’s a kind of reference electrode, and is based on reactions between mercury(I) chloride (calomel) and elemental mercury.
A calomel electrolyte should be a non-polarizable one and robust, they are commonly used two-electrode systems, where the supporting electrolyte is a non-reactive chloride salt.
The structure of the electrode includes of an outer glass with a frit at the bottom, which allows electric contact with a solution outside the electrode. An inner tube is present inside an outer tube. The bottom of the inner tube has glass wool to enable electric contact between the contents of both tubes.
On the innermost tube, is the Mercury paste, with mercurous chloride being dispersed in a saturated potassium chloride solution.
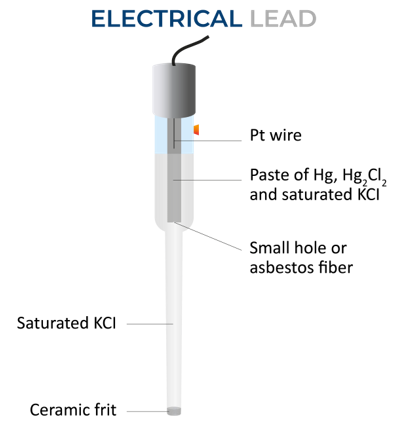
Diagram of a calomel electrode.
It’s a type of Half-cell, where the electrode is mercury that is coated with calomel (Hg2Cl2) and a solution of potassium chloride and saturated calomel forms the electrolyte. This can be represented as:
Hg|Hg2Cl2KCl (xM) saturated
The electrode reaction is:
Hg2Cl2 + 2e Hg2Cl2 == 2Hg + 2Cl
This electrode is an ion selective electrode that is made of doped glass membrane, and the membrane is sensitive to a specific ion. The ion selective glass electrode is extensively used to measure pH. The pH electrode is a good example of the glass electrode that is sensitive to the hydrogen ions. These electrodes also help in the instrumentation of physio-chemical and chemical analysis. The voltage of the glass electrode, relative to some reference value, shows sensitivity to changes in the activity of certain ions sensitive to change.
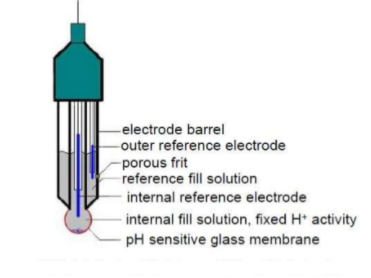
Diagram of a glass electrode
PH is the measure of the hydrogen ion concentration in a solution. It describes the degree of acidity or alkalinity (basicity) of a solution. pH measurement is used most often industrially with, but not limited to, aqueous (water-based) applications. The units of measure scale in most common applications ranges from 0 to 14 pH. 0 pH represents 1 (100) mole/liter of hydrogen, while 14 pH represents 0.0000000000001 (10-14) moles/liter of hydrogen. There is a factor of ten difference between each pH unit.
To determine the Ph of a solution using Glass electrode, a reference solution of known pH is estimated using two electrodes, a reference and a glass electrode. The difference in the potentials generated by both the electrodes is estimated. The thin glass membrane creates an electromotive motive force due to the differences in pH present in the solutions both inside and outside the thin glass membrane, this electromotive force is in proportion to the difference in pH. This thin membrane is known as the electrode membrane. Normally, when the temperature of the solution is 30 ℃, if the pH inside is different from that of outside by 1, it will create approximately 60 mV of electromotive force.
The pH of the liquid inside the glass electrode is 7, thus the emf that is generated at the electrode membrane can be easily calculated. The liquid inside the glass electrode usually has a pH of 7. Thus, if one measures the electromotive force generated at the electrode membrane, the pH of the sample can be found by calculation.
So, pH = 9Ecell – Ecalomel + Eglass) / 0.0591
A second electrode becomes necessary to measure the emf of the glass membrane present in the glass electrode, the other reference electrode that is used should have a stable potential Therefore, it is provided with a pinhole or a ceramic material at the liquid junction.
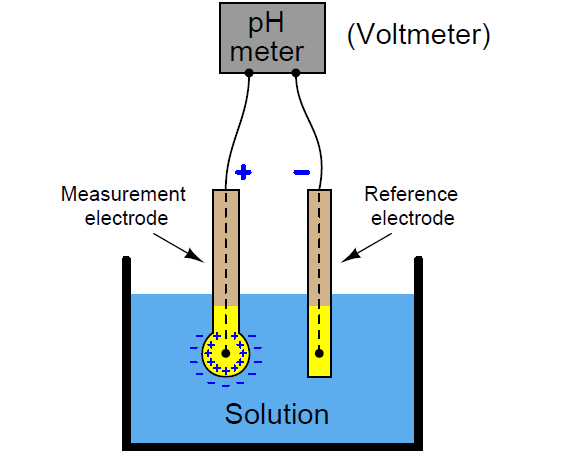
In other words, a glass electrode is devised to generate accurate electromotive force due to the difference in pH present in the solution. And a reference electrode is devised not to cause electromotive force due to a difference in pH.
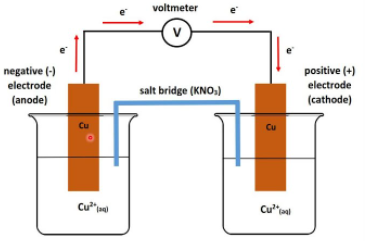
The electrolyte cells have similar electrodes that are immersed in the solution with same electrolyte, but the electrolyte has different concentrations. In these cells the electrolytes tend to move from a higher concentrated solution towards a lower concentration.
An example for this type of cell is a cell where the anode consists of Zn/Zn2+(0.1M) whereas the cathode consists of Zn2+(0.01M)/Zn. In this cell, the flow of electrons from the anode to the cathode is due to the reduction of Zn2+ ions at the cathode into metallic zinc.
Energy storage is the energy that is stored, the energy is captured and stored for later use. This helps in reducing imbalances between energy production and energy demand. A battery is generally the device that stores energy. The storage of energy helps in converting the energy form that may be difficult to store to more convenient and stable form.
A Battery is an energy storage device that contains one or more electrical cells, these cells convert chemical energy to electrical energy. Each battery is a galvanic cell, a redox reaction occurs here between two electrodes which act as the source of chemical energy.
Primary Batteries: The simplest way to understand primary batteries is that they are used only once and are discarded after a single use. Primary batteries are also called non-rechargeable batteries, as they cannot be recharged and used again. These types of batteries have a wide range of advantages that make them the first choice for most of the users. First of all, primary batteries cost super low as compared to other smart batteries. Besides affordability, these batteries are easy, simple, and convenient. This property makes them super reliable durable and available in a wide range of sizes and shapes.
Secondary Batteries: they are also known as rechargeable batteries, these batteries come with electrochemical cells, where chemical reactions came easily be reversed when same amount of voltage is applied in an opposite direction. Secondary batteries can be recharged and used again; they are applicable in high drain appliances. They are used in hearing aids, wristwatches, telephone exchange, computers etc.
Reserve Batteries:
- A reserve battery, is a primary battery and also known as stand-by battery, in such batteries part of it is isolated till the usage of the battery. Reserve batteries are used when long storage is required. Since the active chemicals of the cell are segregated until needed, thus reducing self-discharge. They are commonly used in radiosondes, bomb fuses, missiles and various weapon systems.
It is a type of rechargeable battery, where a practical replacement of Ni-Cd to Nickel–metal-hydride (NiMH) takes place. Ni-MH forms the anode instead of Ni-CD. The Ni-MH uses nickel oxyhydroxide (NiOOH) as the positive electrode., and negative electrode uses hydrogen absorbing alloy instead of cadium. The electrochemistry of the rechargeable Ni–MH battery is given below:
Negative electrode reaction:
H2O+M+e−⇌OH−+MH
The discharge reaction is red from right to left and the charge reaction is read from left to right
Positive electrode reaction:
Ni(OH)2+OH−⇌NiO(OH)+H2O+e−
Nickel oxyhydroxide, NiO (OH), is formed.
Ni–MH is known to generate high power peaks and has less effect on memory, with less toxic chemicals and higher specific energy. It also has good discharge and also is environmentally friendly. On the other hand, they are very expensive, and has higher self-discharge but have a lower efficiency than the lead–acid and Ni–Cd batteries. Ni–MH is available for AA and AAA batteries and is used to power small appliances including medical instruments, cell radios, and other small appliances.
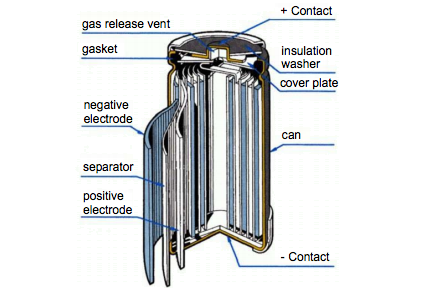
Construction of Ni-MH battery
Lithium-ion batteries predominantly have four components, two electrodes (cathode, anode), separator and electrolyte. The two electrodes and separator porous in nature and gets filled with the electrolyte. The electrodes present conduct electrons, they are separated by the separator electrically, as the electrodes conduct electrons, they reach the current collectors on which electrodes are coated.
During the preparation of the electrodes, carbon black is added to increase the electric conductivity and a binder is added to for hold all the electrode tightly to the current collector.
The main electrochemical activity that takes place in the cell is oxidation/reduction at the electrodes, during the reaction lithium ions are taken or released. In an oxidation reduction process, at one electrode oxidation takes place then at the other reduction takes place and thereby electrons also being released as a by-product, this way current is obtained. During the oxidation and reduction process, ions need to move between the electrons, this task is fulfilled by the electrolyte that consists of plenty of ions that move between the electrons while the separator stops the flow of electrons between them from inside.
While discharging the lithium ions move from anode to cathode since always ions which are negatively charged always move towards higher potential that is cathode, while charging we bias the anode towards higher potential thereby ions reverse it path back to anode. The cell potential is determined by the difference between the chemical potential of the lithium in the cathode and anode.
Oxidation and Reduction Reactions
Oxidation and reduction always occur in pair, for example in a battery with LiCoO2 as cathode and Carbon as anode, in discharging condition we have (oxidation is loss of electrons while reduction is gain of electrons)
In terms of chemistry, the equations can be written as follows
Reduction at cathode: CoO2+Li++e−→LiCoO2
Oxidation at anode: LiC6→C6+Li++e−
The complete reaction would be: LiC6+CoO2→LiCoO2+C6
A lithium-ion battery or Li-ion battery, are batteries that are rechargeable. They are popular in aerospace and in military, they are also commonly used in portable electronics and electric vehicles.
In the batteries, the lithium ions, during discharge move from the negative electrode to the positive electrode through an electrode. And back while charging. At the positive electrode the intercalated lithium compound is present, and graphite at the negative electrode, the battery shows high energy density, low discharge and no memory loss. They can however be a safety hazard since they contain flammable electrolytes, and if damaged or incorrectly charged can lead to explosions and fires.
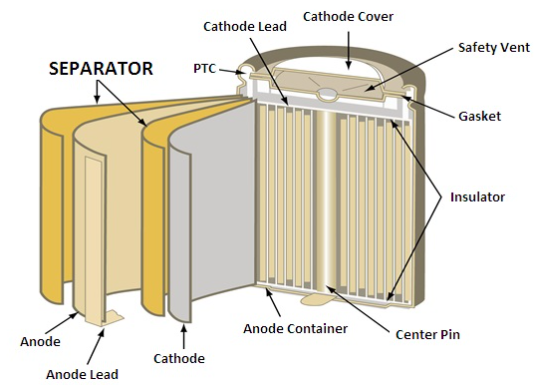
Construction of a Li-ion Battery
References:
1. O.G. Palanna, "Engineering Chemistry", Tata McGraw Hill Education Pvt. Ltd. New
Delhi, Fourth Reprint (2015- Edition).
2. R.V. Gadag & A. Nityananda Shetty., "Engineering Chemistry", I K International
Publishing House Private Ltd. New Delhi (2015- Edition).
3. "Wiley Engineering Chemistry", Wiley India Pvt. Ltd. New Delhi. Second Edition-
2013.
4. B. Jaiprakash, R. Venugopal, Sivakumaraiah and Pushpa Iyengar, Chemistry for
Engineering Students, Subhash Publications, Bengaluru, (2015-Edition).