Unit-2
Corrosion and Metal finishing Corrosion
Corrosion is the disintegration of a metal due to the chemical reactions between the metal and the surrounding environment. Both the types of metal and the environmental conditions, particularly gasses that come in contact with the metal, determine the form and rate of the corrosion.
This corrosion occurs in metals that come in direct contact with a conducting liquid of two different metals are dissolved in a solution partly. There is formation of galvanic cell on the metal, as part of the metal acts as an anode and the rest is the cathode, the chemical in the solution along with the humidity acts as the electrolyte. Oxidation takes place in such conditions resulting in corrosion at the anode surface and reduction occurs at the cathode surface of the metal. In this case corrosion occurs at the anode but rust gets deposited on the cathode.
- Ratio of Anodic and cathodic areas
The rate of corrosion is influenced by relative size of cathodic to anodic area. When a metal is taken into consideration, corrosion rate is very high if the anode area is small and the cathode area is large, as this ratio decreases the corrosion rate increases further. The reason, the electrons are liberated at the anode and are consumed by the cathode region, if the cathode region is large the electrons liberated are consumed rapidly consumed at the cathode, further this enhances the anodic reactions that further increases the overall rate of corrosion. When two dissimilar metals are in contact, Corrosion is more rapid and severe, if the anodic area is small and cathodic area is large (e.g., a small steel pipe fitted in a large copper tank), If during plating of tin on iron, some areas are not covered or some pin holes are left, there results a small anode and large cathode.
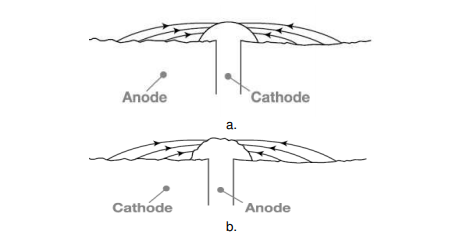
Figure. Effect of area ratios of bimetallic corrosion a. Large anode area, small cathode area showing relatively insignificant attack over a wide area of the sheet. b. Large cathode area, small anode area showing relatively pronounced attack of the rivet head.
- Nature of corrosion product
The nature of the corrosion product like metal oxide may serve as a protective film, if the product is stable, insoluble and nonporous. The protective film can further prevent corrosion by acting as barrier between metal surface and corrosion medium. On the other hand, corrosion is enhanced if the product is unstable, porous, and soluble.
E.g., in oxidizing environments, metals that form protective layers are aluminium, chromium, titanium, etc., are highly passive and protect the metal surface from further corrosion. On the other hand, metals such as iron, zinc, magnesium etc., do not form any protective film and are highly susceptible for continuous corrosion, when exposed to oxidizing environments.
pH of the medium
Generally, corrosion is high in acidic pH than in alkaline or neutral medium, in Iron a protective layer of iron oxide is formed at a very high pH. In low pH, however severe corrosion occurs. Corrosion rate is very high at higher ph in metals like Aluminium.
- Temperature:
With the increase in temperature the rate of corrosion increases, as the temperature increases there is an increase in the conductance of the medium hence the diffusion rate also increases. In some cases, however the increase in temperature decreases passivity, which again leads to increase in temperature, hence with the increase in temperature the rate of corrosion increases. - Conductivity
Corrosion does not occur in distilled water, as the conductivity increases the rate of corrosion also increases, due to the presence of more ions in the solution. Fresh water allows corrosion of steel to a less extent than brackish water or estuary water, sea water is usually most corrosive to steel.
Differential Metal
When two different or dissimilar metals come in contact with each other and have different electrode potential values, when both these metals are exposed to a corrosive environment. The metal with lower electrode potential value forms the anode and undergoes oxidation and corrosion takes place, whereas the metal with higher electrode potential value forms the cathode and remains unaffected.
Pitting corrosion
Pitting corrosion, or pitting, is a localised corrosion, where small holes are formed on the metal surface, in this small area depassivation occurs that becomes anodic and the rest of the vast area becomes the cathode, oxidation occurs at the anode and reduction at the cathode, leading to a localised galvanic corrosion. The corrosion penetrates the mass of the metal, with a limited diffusion of ions.
Waterline corrosion
It occurs when the differential oxygen concentration differs from water and the atmosphere, this occurs as the portion of the substrate exposed to more amount of oxygen (air) becomes the cathode, while the portion of the substrate expose to less oxygen (water) forms the anode, resulting in oxidation reaction. This ultimately causes the area of the substrate submersed in water to become oxidized and corrode.
Differential Aeration
Differential aeration corrosion is a type of corrosion, where the metal surface show varying concentrations across its surface. The area with lower oxygen concentration forms the anode and becomes the portion that is subjected to corrosion, the area with higher concentration becomes the cathode
Some examples where varying concentrations of oxygen may be found are metals that are buried, certain joint types, crevices and cracks.
Anodizing of Aluminium
Anodizing is an electrolytic surface treatment most commonly used with aluminium components. This treatment provides durable, hard, corrosion resistant and nonconductive finish on the outer surface of the anodized part. The oxide layer is porous in nature and makes the surface of the component easy to dye and paint. For this reason, it is often used as a pre-treatment for parts that will be dyed, coated or bonded
Anodizing is accomplished with the component or workpiece submerged in a tank filled with an electrolytic acid solution. A current (typically low, from 5-20V) is passed through the solution via a cathode submerged in the solution, with the workpiece serving as the anode. Oxygen is released by the current at the surface of the aluminium, building up a layer of aluminium oxide on the outside of the part.
Cathodic Protection
Cathodic protection in this process the main principle is to switch from the works by changing over undesirable anodic (active) sites on to a metal's surface to cathodic (passive) destinations in the presence of a restricting current. This restricting current provides free electrons and also supplies power to the nearby anodes,
Cathodic protection can, the presence of galvanic anodes. This process is also known as conciliatory form, this technique utilises metal anodes together with the electrolytic condition, to make themselves strong (erode) so as to secure the cathode. This technique proves to save the metals galvanic corrosion.
Though the metal that requires protection can differ, the conciliatory anodes are largely made up of magnesium, or zinc, metals that have the most negative electro-potential. The arrangement of galvanic setup gives a glimpse of the distinctive electro-potential - or integrity - of metals and amalgams.
In a conciliatory framework, the anode should be supplanted regularly as the metallic particles move from the anode to the cathode, which drives the anode to erode more rapidly. The second technique for cathodic security is alluded to as awed current protection.
This strategy, which is regularly used to ensure covered pipelines and ship bodies, requires an elective wellspring of direct electrical current to be provided to the electrolyte.
Sacrificial Anode
It is a method where an easily eroded material is deliberately placed in a tank or a pipe to be sacrificed for corrosion and the rest of the system remains free from corrosion.
A sacrificial anode is also known as a galvanic anode
The mechanism that occurs in this process is very similar to that of an electrochemical system. In this technique the metal that is protected is placed on the cathode side and then a more reactive metal or alloy (having a larger potential difference than the protected metal) is chosen and connected to the protected metal as an anode. The redox reaction will proceed spontaneously. As the reaction proceeds the sacrificial metal gets consumed as oxidation reaction occurs at the anode, simultaneously reduction reaction occurs on the cathode, thus preventing the metal from erosion. Thus, corrosion on the protected metal is successfully shifted to the anode, protecting the metal.
Sacrificial anodes are normally supplied with either lead wires or cast-m straps to facilitate their connection to the structure being protected. The lead wires may be attached to the structure by welding or mechanical conn
The materials used for sacrificial anodes are either relatively pure active metals, such as zinc or magnesium, or are magnesium or aluminium alloys that have been specifically developed for use as sacrificial anodes.
Advantages of using sacrificial anodes:
- Can be used where there is no power
- Lower initial cost
- Less supervision required
- Comparatively simple installation and additional anodes can easily be added if the initial installation proves to be inadequate
Sacrificial anodes are used to protect:
- Hulls of ships
- Water heaters
- Pipelines
- Distribution systems
- Above-ground tanks
- Underground tanks
- Refineries
The anodes in sacrificial anode cathodic protection systems must be periodically inspected and replaced when consumed.
Impressed current methods
In this technique, protection from corrosion is obtained by electrochemical means. It is a type of cathodic protection, where the dissolution of anodic structures is reduced, that occurs by decreasing the difference the potential energy, and also when the metal surface is placed in a conductive electrolyte such as water and the cathodic and anodic metal surface sites face less reduction.
Theoretically, on observation the impressed current cathodic protection is obtained during the stage where open circuit potential of cathodic areas gets polarized into the same circuit potential of anodic sites. The key in impressed current protection is to turn the whole structure cathodic in nature, or make it a current receiver rather than a current provider.
An impressed current protection system can offer protection to structures and metals like:
- Fuel pipes
- Steel
- Water
- Hulls
- Tanks
- Water heaters
- Oil casings
It can also serve as a metal reinforcement in the case of concrete buildings, structures and bars. The method can be applied in galvanised steel, where the steel parts are protected from rusting by sacrificial zinc coatings.
Utilizing this type of protection offers the following practical benefits:
- Infrastructure cost for corrosion is reduced.
- Infrastructure failure incidents become rare.
- Product loss from corrosion is reduced.
- Lower downtime in terms of emergency repairs
- Increased service life of infrastructures
- Enhanced quality of infrastructure service
- Increased security and health to underlying infrastructures
An impressed current system serves as an ideal protection system for bigger structures that are not capable of delivering ample current to offer protection. This system is mainly composed of anodes attached to a power source (DC), which can be a rectifier connected to power (AC). In the absence of an AC power supply, other power sources may be used like wind power, solar panels and gas power. The anodes in the impressed current protection system can be found in various sizes and shapes. The most common ones are tubes and solid rods, while there are also continuous ribbons made from various materials like:
- Platinum
- Graphite
- Silicon
- Cast iron
Metal-coating
- Galvanizing, it is a process that involves the protection of steel and iron from exposure to the atmosphere leading to rusting, this is however prevented by the application of zinc coating. Proper coating by galvanization can protect the material from the atmosphere and corrosion for nearly 15 to 30 years, however is pores develop in the coating galvanic or electrolytic action occurs.
In the manufacturing process this forms the final step, this process provides environmental protection. This is also used to reduce the roughness of the surface beyond the capabilities of machining operations for parts that must mate or seal. The other processes also include metal cleaning, deburring, descaling etc, numerous types of metal finishing process are used for a range of purposes. Some of the general advantages of metal finishing treatments include:
- To Increase durability
- To Improve decorative appeal
- Enhanced electrical conductivity
- Higher electrical resistance
- Higher chemical resistance
- Higher tarnish resistance
Electroplating is basically the process of plating a metal onto the other by hydrolysis, that helps to prevent corrosion of metal. The method used is electric current that reduces dissolved metal cations, that forms a thin coherent metal coating on the *electrode. The process uses an electric current to reduce dissolved metal cations to develop a lean coherent metal coating on the electrode.
Polarization: During electrolysis, the potential of the electrode changes due to Polarization, where the anode potential becomes nobler than that of cathode. Polarization has the property of decreasing the output voltage of batteries based on the conditions and thereby increases the voltage that is required for electrolysis cells.
This process is also known as kinetic deviation from the equilibrium because of an electric current passing through a galvanic cell. Polarization may occur at the cathode (cathodic polarization) or at the anode (anodic polarization). Cathodic polarization is more common
Decomposition Potential
A decomposition potential, in electrochemistry, is the difference in the electrode potential between an electrolytic cell's cathode and anode in order for electrolysis to occur. It is the minimum voltage produced from an electrolytic cell and is used in the electrolysis process.
Overvoltage
Overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency.
The corrosion resistance is beat achieved when decorative chromium gets deposited on the surface that is coated with copper and nickel.
The undercoating of Copper is deposited by copper strike and is followed by copper electroplating. Copper helps in covering the defects of the substrate surface and also protects the surface from corrosion, copper also helps in improving the decorative appearance.
The undercoating of Nickel is deposited by Nickel electroplating in one or two layers. Nickel helps in levelling the substrate surface and also provides corrosion resistance, in addition Nickel also helps in surface preparing for chromium plugging.
Decorative chromium is deposited directly on bright underlying nickel.
The decorative chromium thickness lies in the range of 0.01-0.03 μinch (0.25-0.75 μm).
In decorative chromium numerous micro cracks and micropores are covered by under coating of Nickel, these microdefects play an important role in providing cathodic protection for the undercoating of nickel. Since the micro-cracks or micro-pores are uniformly spread over the chromium surface, corrosion reaction is not localized and therefore it proceeds slowly.
Hard chromium (chrome): It’s a relatively thick coating that is deposited on the metallic substrate mostly steel, in order to impart few specific properties to the surface.
The main component of all the chromium plating solution is chromium trioxide (CrO3) also known as chromic acid. The second component is a catalyst, which is either sulfate (SO42-) or fluoride.
Trivalent chromium (Cr3+), is the by-product of the electroplating process. Ions of trivalent chromium continuously reoxidize to the hexavalent state at the anode. Normal level of the trivalent chromium is about 1-2% of the chromic acid concentration. Higher contents of trivalent chromium may cause reduction of throwing power and plating rate, pitting and treeing of the deposit. If the trivalent chromium is too high (more than 2%) reoxidation operation should be carried out at high anode area/cathode area ratio (30) at cathode current density 20 A/ft² (2 A/dm²).
This process was discovered by A. Brenner and G. Riddle in 1944. This involves the plating of metals and does not use electricity. This is achieved by a process of controlled autocatalytic reduction that gives a uniform coating to plastics and metals. The principle of this method is the deposition of metals such as copper, silver, nickel and gold that is present on a variety of material by means of a chemical bath. This process is also used in mirroring, wherein a clear piece of glass is dipped in an ammoniacal silver solution mixed with Rochelle salt or with a nitric acid–cane-sugar alcohol solution. Non-metallic surfaces, such as plastics, must be chemically treated prior to electroless plating.
This is the most effective and simplest way of applying a nickel plate over steel, copper, iron, brass, zincated aluminium and copper alloys.
The Nickel is applied by heating the Nickel bath to 195°F and then dipping the part to be plated in the bath for 15-60minutes, this process does not require DC power, and the process time is carried till the desired thickness is observed. In nickel coating an autocatalytic reaction occurs that applies the nickel coating.
The tricky part about electroless plating is that you must keep at least 80% of the nickel in the plating solution at all times. Letting the amount of nickel get below 80% will cause the bath to crash, rendering it useless.
5.12 Electroless copper plating
In solid substrates such as metal or plastics, the deposition of an even layer of copper occurs. The process involves immersing the solid substrate in a water solution containing copper salts and a reducing agent like formaldehyde.
No external current is required for the bath or the substrate, the reduction of the metal cations that are present in the solution is achieved by chemical means through an autocatalytic reaction. The method can also be applied to non-conductive surfaces, thus electroless plating creates an even layer of metal regardless of the geometry of the surface.
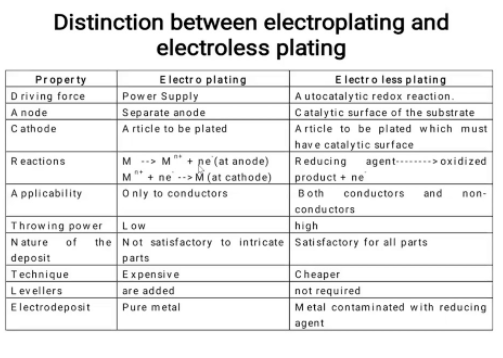
5.13 distinction between electroplating and electroless plating processes
References:
1. O.G. Palanna, "Engineering Chemistry", Tata McGraw Hill Education Pvt. Ltd. New Delhi, Fourth Reprint (2015- Edition).
2. R.V. Gadag & A. Nityananda Shetty., "Engineering Chemistry", I K International Publishing House Private Ltd. New Delhi (2015- Edition).
3. "Wiley Engineering Chemistry", Wiley India Pvt. Ltd. New Delhi. Second Edition-2013.
4. B. Jaiprakash, R. Venugopal, Sivakumaraiah and Pushpa Iyengar, Chemistry for Engineering Students, Subhash Publications, Bengaluru, (2015-Edition).