Unit-3
Energy Systems Chemical Fuels
A fuel is a material which can react with other substance to release energy in the form of heat energy, the energy is otherwise used to do work. Initially this concept was applied only to those materials that are able to produce chemical energy, but has now applied to other sources of heat energy like nuclear energy.
Fuels release heat energy that is converted to mechanical energy through heat engine, during other times the heat energy for cooking or for industry processes, or the illumination that comes from combustion. The fuels are also used in cell organisms in a process called cellular respiration, where oxidation of organic molecules occur to release energy. The most source of fuel used by humans are hydrocarbons and related oxygen molecules, but other substances are also utilised, including radioactive metals.
Classification of chemical fuels
Chemical fuels are divided in two ways. First, based on their physical properties, as a solid, liquid or gas. Secondly, on the basis of their occurrence: primary (natural fuel) and secondary (artificial fuel). Thus, a general classification of chemical fuels is:
General types of chemical fuels | ||
| Primary (natural) | Secondary (artificial) |
Solid fuels | Coal, peat, dung wood etc. | Coke and charcoal |
Liquid fuels | Petroleum | Diesel, ethanol, naphtha, diesel tar, coal gasoline, kerosine |
Gaseous fuels | Natural gas | Coke, gas. CNG, propane, methane water gas. Hydrogen, oven gas, coal gas. |
Solid fuel refers to different types of solid material that are used as fuel to produce energy and provide heating, that is released by combustion., Solid fuels include fuel tablets, wood, charcoal, peat and pellets made from wood, rye, grains, wheat. Solid rocket fuel technology also uses solid fuel. Solid fuels have been used by humanity for many years to create fire
Liquid fuels need to take the shape of the container, the fumes of liquid fuels are flammable. These fuels are combustible or energy-generating molecules that can be used to produce mechanical energy usually kinetic energy.
Most of the liquid fuels are produced from the fossil remains of the dead animals and plants when exposed to the heat and pressure inside the Earth’s crust, there are however other types of liquid fuels like hydrogen fuel, jet fuel, ethanol and bio-diesel.
Fuel gas is in gaseous form under ordinary conditions, many fuels are composed of hydrocarbons (like methane and propane) carbon monoxide, hydrogen or may exist in the form of mixtures. Gaseous fuels are potential sources of heat and light energy. And can be distributed and transmitted through pipes from the beginning to directly to the place of consumption. Fuel gas is contrasted with solid and liquid fuels, though some fuel gases are liquified for storage purposes
Calorific Value
It is the heat liberated by the combustion of the fuel, the heat required to evaporate the water should be neglected, is called the High Calorific value. It is the maximum heat liberated by complete combustion of fuel; HCV is also called gross calorific value.
Calorific value of fuels
The calorific value is the value that shows the amount of heat liberated on complete combustion. For solid and liquid fuels, calorific value is expressed in kJ/kg, whereas for gaseous fuels it is expressed as kJ/m3 where m3 is normal cubic metre measured at NTP conditions i.e., at 0°C temperature and 760 mm Hg barometric pressure (1.01325 bar). Sometimes, calorific value of gaseous fuels can also be expressed as kJ per cubic metre expressed at STP conditions. STP (standard temperature and pressure) conditions are taken as 15°C and 760 mm Hg barometric pressure (1.01325 bar)
High Calorific value (HCV)
It is the heat liberated by the combustion of the fuel, the heat required to evaporate the water should be neglected, is called the High Calorific value. It is the maximum heat liberated by complete combustion of fuel; HCV is also called gross calorific value.
If we subtract from the higher calorific value, an amount of heat required to evaporate the water formed, we get Lower Calorific Value (LCV) or Net Calorific Value (LCV) or Net Calorific Value of the fuel.
No definite agreement is to be found in the literature on fuel as to whether the lower calorific value shall be found simply by subtracting latent heat of steam or both the latent heat and sensible heat in cooling from 100°C, from the gross calorific value of the fuel; in the latter case fixing the temperature becomes mandatory to which the products are finally reduced. In literature the net calorific value of the fuel is obtained by subtracting from higher calorific value the amount 2466 kJ/kg (latent heat of dry and saturated steam at STP (15°C) conditions) (amount of water formed because of the combustion of 1 kg fuel.)
Basically, a bomb calorimeter consists of a small cup to consists of the sample, Oxygen, A stainless steel bomb, Water, a stirrer, a thermometer, and the Dewar (to prevent heat flow from the calorimeter to the surroundings) and Ignition circuit connected to the bomb.
Two electrodes are connected to each other through platinum wire.
• The wire remains in a platinum dipped cap just below it.
• A small amount of substance under investigation is taken in platinum cap. The vessel is then filled with excess of oxygen at a pressure of about 20-25 atm and is subsequently scaled.
• It is now dipped in an insulated water bath which is also have mechanical stirrer and a thermometer.
- It is now dipped in insulated water bath, this also has a mechanical stirrer and a thermometer
- The initial temperature of water is noted, combustion is started by passing electric current through the platinum wire, resulting in increase in temperature of water, the increase in temperature is noted
- Thus, by knowing the heat capacity and also raise in temperature the heat evolved can be calculated.
A sample of coal has following composition by mass C = 70 %, O = 8 %, H = 10 %, N = 3 %, S = 2%, Ash = 7 %. Calculate H.C.V. And L.C.V. Using Dulong formula [Difficulty level-high]
Solution: HCV = 1/100[8080 C+34500(H-O/8) +2240 S] = 1/100[8080 + 70+34500(10-8/8) +2240 X 2] = 1/100 [565600 + 34500 (10-1) X 4480] = 1/100 [565600 + 310500 + 4480] = 1/100 [880580] = 8805.80kcal/kg LCV = [ HCV- 9/100 H X 587] kcal/kg = [8805.80-9/100 x 587] = [8805.80- 528.30] = 8277.80 kcal/kg
Answer: HCV/GCV = 8805.80kcal/kg
LCV/NCV = 8277.80 kcal/kg
Knocking usually occurs in an internal combustion engine, where sharp sounds occur due to the premature combustion from the part of the compressed air-fuel mixture present in the cylinder. When an engine functions smoothly in the combustion chamber, the charge burns with the flame front smoothly progressing from the point of ignition. In some cases, depending upon the composition of the fuel, at high compression ratios, some of the charge may ignite spontaneously ahead of the flame front resulting in burning in an uncontrolled manner that produces pressure waves of high frequency. The waves are responsible for forcing parts of the engine to vibrate and produce audible sound.
The effects of Knocking can cause overheating of the spark-plug points, erosion of the combustion chamber surface, and rough, inefficient operation.
Prevention
- Addition of 1% nitrate or any nitrate can accelerate the combustion of fuel. This is known as doping and helps in delay period considerably.
- Higher temperatures are required for spontaneous ignition of the fuel, this is achieved by raising the compression ratio., a rise in pressure also helps in smooth running of the engine.
- By increasing the turbulence of the compressed air, it promotes homogeneous mixture by stripping the fuel from the spray.
- The fuel injector should be arranged in such a way that it starts injecting only a small quantity of fuel to start the engine. Atomization also occurs when the injection pressure is increased that prevents knock in engines.
Power alcohol is a mixture liquid. It is found in automobiles; they are known to generate energy from their own resources. Power alcohol is a good fuel and is obtained by mixing 75% petrol and 25% ethyl alcohol
Unleaded Petrol: a small amount of lead is added to fuels, so that they produce less harmful substances when burnt. Lead that is added is mainly tetraethyllead that has a low knocking factor. The lead that is added to petrol does not have any additives.
Biodiesel: is mainly obtained from oily plants, algae, animal fat and waste cooking oil, the biodiesel is also blended with petrol and diesel fuels in various percentages. The common plants that are used in biodiesel production are soybean and oil palm.
Fuel cells are electrochemical cells that generate electric energy from fuel through an electrochemical reaction. These cells need a continuous input of fuel and an oxidizing agent which is mainly oxygen. This continuous input is required to sustain the reaction as they produce electricity. Fuels cells can therefore generate electricity constantly until fuel is supplied, fuels cells consist of an anode and a cathode and an electrolyte which enables movement of protons.
Fuel cell | Battery |
A Fuel cell is a device that can convert chemical energy into electrical energy | A battery is device containing two or more electrochemical cells that can convert chemical energy into electric energy directly. |
Can provide us the electrical energy for a longer period of time. | Produces electrical energy for a comparatively short time period. |
Continuosly supplied with fuel and oxygen from an external source which makes it work for a long time period | Contains a limited amount of fuel and oxidant and these two components decrease with time.so this device cannot supply electrical energy for a long period of time |
Limitations
- High flammability leads to an explosive quality to the fuel-air mixture.
- Leak detection is difficult as it is odorless.
- On board storage is difficult due to low energy volume density.
- (Huge storage space is required in compared to other fuels)
- Pre ignition and backflash causes engine design challenges.
- Expensive as production quantity is limited.
- Lack of distribution infrastructure.
Advantages of fuel cells
The big advantage of hydrogen fuel cells is that the only emission they produce water vapour.
Hydrogen has low density, making vehicles light and efficient.
Hydrogen gives out more energy per gram than conventional fuels.
Disadvantages of fuel cell technology
Production of hydrogen from fossil fuels produces carbon dioxide – greenhouse gas.
Hydrogen is highly flammable, making it risky to store.
Hydrogen is not cheap!
The cell consists of mainly of anode and cathode compartments, both these compartments contain the platinum electrode. Platinum electrode is present in both the compartments, The Electrolyte is sulphuric acid, oxygen is passed through the cathode and methanol containing sulphuric acid passes through the anode. A membrane is present that prevents the diffusion of methanol into the cathode.
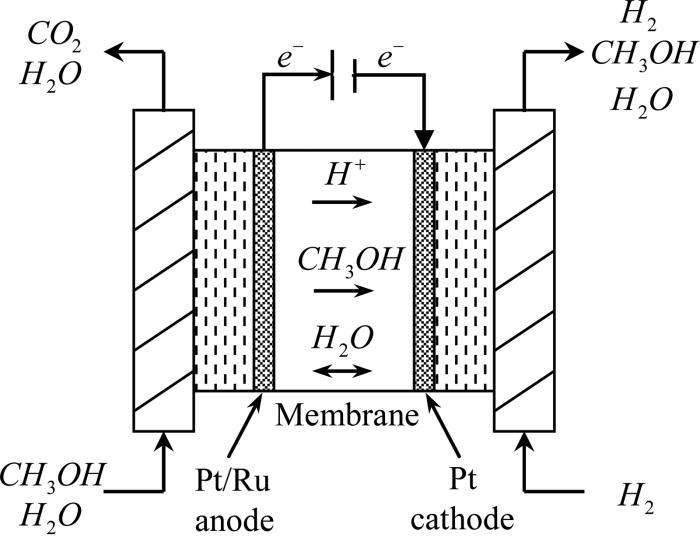
Reactions:
At anode: CH3OH + H2O → CO2 + 6H+ + 6e-
At cathode: 3/2 O2 + 6H+ + 6e- → 3H2O
The advantage of using methanol is they have a very low carbon content and highly soluble in water.
Application
These cells can store high energy in a very small space
They are very useful in military applications, as they have no toxic effluents, low noise and low thermal signatures
Other applications include power for man-portable tactical equipment, battery chargers, and autonomous power for test and training instrumentation.
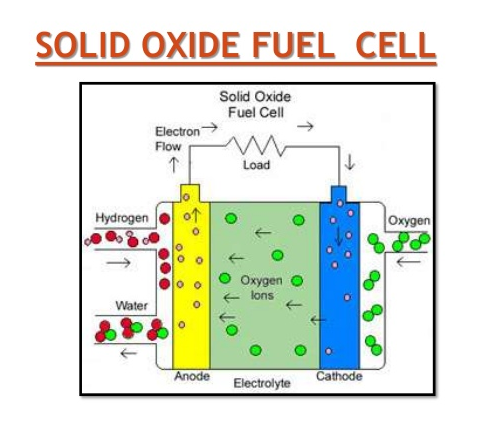
This is the most commonly used fuel cell, as they have the highest operating life compared to the others, the operating temperature is 1000oC.when used in a continuous state these cells are highly reliable, but may be unstable if other parts of the cell are unable to withstand the extreme temperatures. At high temperatures, water is produced in the form of steam that is easily transported through steam turbines, that help in producing more electricity thereby increasing the efficiency of the system. The electrolyte used in the cell is good for large scale power generation and has few characteristics as other electrolytes, it is called (YSZ) Yttria Stabilised Zirconia.
However, there are few disadvantages, due to the extreme temperatures, a few reactions may take place that may not be necessary, as a result of these reaction Dust and graphite may pile up at the anode making it insufficient to react with the catalyst.
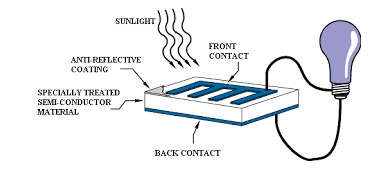
Photovoltaic cell
- These cells contain two or more layers of semiconductors, the two layers are adjacent to each other, with one layer having a positive charge and the other layer a negative charge.
- The Sunlight contains small pockets of energy called as photons, these photons strike the cells, resulting in either small or big transitions.
- The energy of the photon gets transferred to an electron present in the atom of the cell, when the photons are absorbed in the negative layer of the cell.
- As the energy increases, the electron escapes from the outer shell of the atom, this electron migrates to the positive layer of the photovoltaic cell thereby creating a potential difference between the positive and negative layers, when these two layers are connected to an external circuit, current is produced.
.
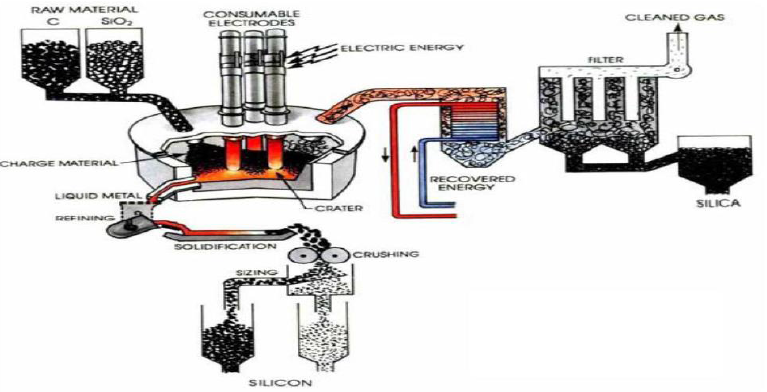
Preparation of Solar grade Silicon by Union Carbide
In this method Quartz and coke are placed in the vessel, submerged electrical arc furnace, the Solar grade Silicone is a compound that lies between the metallurgical grade and the semiconductor grade. A very temperature of 17000OC, is struck in the arc where carbothermic reduction takes place, SiO2 is reduced to Si.
SiO2(s) + 2C(s) Si(l) + 2CO(g)
The above liquid crude silicon contains 1 to 3% impurities. So that’s why crude silicon is treated
With O2 and silica sand (SiO2), impurities like Al, Ca and Mg etc are removed as oxides.
4Al + 3SiO2 3Si(l) + 2(Al2O3)
2Ca + SiO2 Si(l) + 2(Cano)
2Mg + SiO2 Si(l) + 2(MgO)
The liquid silicon is poured into a casting mould and solidified. It is crushed into small lumps (100nm), these lumps are converted to Trichlorosilane (HSiCl3) by HCl (Dry gas)
Treatment at 3500C. The purified HSiCl3 is passed into a fixed bed column filled with quaternary ammonium ion exchange resin as catalyst. It is converted into Dichlorosilane (H2SiCl2). Dichlorosilane is passed through the hydrogenation chamber having a second fixed bed column of quaternary ammonium ion exchange resin, it is converted into Silane (SiH4).
Advantages of Photovoltaic Cells:
- Environmental Sustainability: No harmful gases like CO2 or NO are produced, no noise pollution is produced, therefore they become an ideal application for residential areas and the energy produced is also clean and green.
- Economically Viable: Apart from the purchase and installation of the solar panel, the maintenance and operations cost are very low.
- Accessible: The panels can be easily installed and is easily accessible even in remote areas at a very less cost, compared to the conventional lines, the setup of the solar panels does not disturb the residential lifestyle.
- Renewable: Energy is free and abundant in nature.
- Cost: The solar panel have no mechanical moving parts, except in exceptional cases where the panels are highly advanced sunlight tracking mechanical bases. Consequently, the solar panel price for maintenance and repair is negligible.
Disadvantages of Photovoltaic Cells:
- The efficiency of the panels is low when compared to the other renewable sources of energy.
- The energy from the sun is not regular and is also unpredictable, the energy is also reduced during cloudy weather conditions.
- Solar energy that needs transmission of a Long-range becomes difficult and inefficient to carry out. The current produced is DC and to convert Dc to an AC current may require an extra equipment like the inverter.
- Insurance costs are required to keep the panel safe as they are fragile and can be easily damaged.
References:
1. O.G. Palanna, "Engineering Chemistry", Tata McGraw Hill Education Pvt. Ltd. New Delhi, Fourth Reprint (2015- Edition).
2. R.V. Gadag & A. Nityananda Shetty., "Engineering Chemistry", I K International Publishing House Private Ltd. New Delhi (2015- Edition).
3. "Wiley Engineering Chemistry", Wiley India Pvt. Ltd. New Delhi. Second Edition-2013.
4. B. Jaiprakash, R. Venugopal, Sivakumaraiah and Pushpa Iyengar, Chemistry for Engineering Students, Subhash Publications, Bengaluru, (2015-Edition).