Module-4
Environmental Pollution and Water Chemistry
Primary air pollutants are those pollutants that are directly emitted into the air from sources like natural gas power plants, coal fired power plants, volcanoes, burning of biomass, burning of natural forest fires and many more.
These pollutants effect the environment both directly and also form a precursor of secondary pollutants, examples of primary pollutants include- Sulphur dioxide, nitrogen oxides of dioxide, nitrogen organic matter, particulate matter etc.
- Sulphur dioxide (SO2)
During processes like fossil fuel combustion, metal smelting or oil refining, Sulphur is exposed to very high temperatures leading to the formation of Sulphur dioxide, Sulphur dioxide is toxic at very high concentrations, the most adverse effect to the environment is the formation of Acid rain, where the Sulphur dioxide dissolves in the cloud droplets and oxidizes to form sulphuric acid, that falls to the earth as acid rain, or they may also form sulphate aerosol particles in the atmosphere.
- Nitrogen oxides (includes NO NOx and NO2)
When oxygen and nitrogen react at very high temperatures, either during combustion or lightning very highly reactive gas NOx is formed. This reactive gas is also emitted during fossil fuel combustion and biomass burning.
Through a complicated chain reaction NOx reacts with volatile compounds and carbon monoxide in the atmosphere to form ground level ozone through a complicated chain mechanism, and oxidises to form Nitric acid, that also contributes to acid deposition and aerosol formation.
- Carbon monoxide (CO)
It’s an odourless, colourless gas that is formed by incomplete combustion of carbon in fuel. These gases are liberated during burning of biomass and exhaust from vehicles and industrial processes, carbon monoxide plays an important role in ground-level ozone formation.
Carbon monoxide under low levels can aggravate cardiac ailments and under high exposures can cause nervous system impairment or even death, they also bind to haemoglobin in RBC and reduce the transport and release the oxygen in the body.
- Hydrocarbons
Hydrocarbons are emitted from wide range of sources like industrial processes, fossil fuel combustion and natural emission from fires and vegetation, they also form precursors of aerosol and ground level ozone. Major anthropogenic sources of methane include natural gas production and use coal mining, rice and livestock.
- Particulate matter
Particle pollution also known as particulate matter, is a mixture of solids and liquid droplets that are present in air, some are visible to the naked eye like smoke, soot dirt, dust, while others are detected only on a microscope. Particle pollution includes “inhalable coarse particles” that have a diameter larger than 2.5 micrometres and smaller than 10 micrometres and “fine particles” with diameters 2.5 micrometres and smaller. These particles are available in a range of shapes and sizes and made up of numerous chemicals, some primary particles are emitted from unpaved roads, smoke stacks, construction sites and fires, secondary particles include sulphur dioxide and nitrous oxides emitted from power plants, industries and automobiles.
- Lead
Earlier motor vehicles were the major contributors of lead emission
These days the major sources of lead include metal and ore processing, piston-engine operations on leaded aviation gasoline, lead smelters form the highest air concentrations of lead, other sources include waste incinerators, utilities, paint and lead acid battery manufacturers.
- Mercury
When mercury is released (Hg), it creates a lot of environ mental problems. Both humans and natural sources release mercury. It creates environmental problems. Both humans and natural sources release mercury; 50-90% of the mercury that is present in the environment is produced from humans, coal is also known to release mercury. Re-emission occurs when previously stored mercury is reintroduced into the environment by forest fires or other means, and requires complex modelling techniques to determine how much can be traced back to human emissions
Ozone
Is a layer that is present in the earths stratosphere and has high concentrations of ozone and they protect the earth from the harmful radiations from the sun. The ozone layer is present in the lower part of the atmosphere, they have the capacity to absorb 97-99% of the harmful UV radiations coming from the sun, that can cause damage to the life on the earth, if this ozone layer was absent, many people would have developed skin diseases and their immune systems would have become weak.
Recently however scientists have discovered a hole in the ozone layer due to presence of chlorofluorocarbons, carbon tetrachloride, methyl bromide and hydrochlorofluorocarbons. This has caused a major environmental issue
Ozone layer Depletion
The gradual thinning of the earths ozone layer in the upper atmosphere due to the presence of chemical compounds containing gaseous bromine or chloride from human activities and industries.
The thinning of the ozone layer, present in the upper atmosphere occurs when a chlorine and bromine atoms present in the atmosphere come in contact with the ozone and destroy the ozone molecules, a chlorine molecule can destroy up to 100,000 molecules of ozone these molecules are more quickly destroyed than created. Few compounds are released when chlorine and bromine are exposed to UV light that cause a depletion in the ozone layer.
The ozone-depleting substances that contain chlorine include chlorofluorocarbon, carbon tetrachloride, hydrochlorofluorocarbons, and methyl chloroform. Whereas, the ozone-depleting substances that contain bromine are halons, methyl bromide, and hydro Bromo fluorocarbons.
Chlorofluorocarbons are the most abundant ozone-depleting substance. It is only when the chlorine atom reacts with some other molecule, it does not react with ozone.
Solid waste
Solid wate management is the collection, treatment and disposal of the solid material that is discarded as they are no longer useful, improper disposal of the municipal waste can create unsanitary conditions, resulting in pollution of the environment and outbreaks of vector borne disease spread by mice and insects.
Solid waste included commercial, industrial, residential and also institutional waste, waste can become hazardous as certain types of waste can cause immediate danger to the individuals or the environment when they are exposed to it. Municipal solid waste or Refuse is the non-hazardous solid waste from a community that needs collection of the waste and transporting them to a disposal site. Refuse includes both garbage and rubbish, rubbish is mostly the dry material like rubbish, rubbish or wood, garbage is the decomposable food waste. This waste is decomposable and highly putrescible. Trash is rubbish that includes couches, tree stumps and old refrigerators, they require special collection and handling.
Construction and demolition waste is a significant component of the total solid waste quantities; however, they are inert and non-hazardous and is usually dispose in municipal landfills.
E-Waste
The full form of E-waste is Electronic waste, any electronic items that were once useful but is not used anymore due to certain defects form the E-waste. Annually around 50million tons of E-waste is generated in the whole world, they can become a major threat in the near future if they are not properly disposed or recycled properly. As technology improves, most of the old devices become obsolete when the new ones arrive.
E-waste can be any electrical or electronic goods such as computers, TV, monitors, mobiles, PDA, VCR, CD players. Fax machines, Printers etc. If the electronic items are not properly disposed, they form harmful material such as cadmium, mercury, lead and beryllium, these harmful materials form a major threat as they are not decomposable by themselves. Therefore, it is necessary to recycle them properly and efficiently
There are lots of E-waste, they are classified primarily into
- White goods- This includes home based appliances such as air conditioners, washing machines.
- Brown goods-These are televisions, cameras etc
- Gray goods- Computers, scanners, printers and mobile phones.
Biomedical Waste
Is the waste that includes, samples of blood or tissue that are removed from labs, operation theatres, morgues or any medical facilities., they also include anything that is used to treat patients, hospital gowns and beddings.
Types of Biomedical waste
- Waste that is infectious: Any material that is contaminated with body fluids, including blood, cultures and stocks of infections in labs, infectious waste from infected patients
- Sharp objects like blades syringes scalpels and needles that are disposable
- Pathological wastes: contaminated human organs, fluids, tissues, and also body parts of animal carcasses.
- Pressurized containers
- Pharmaceutical waste including drugs and vaccines that are unused, expired or contaminated;
- Chemical waste including disinfectants, heavy metals in medical devices such as mercury, solvents and reagents that are used in lab preparations and batteries;
- Radioactive waste including radiotherapeutic materials, radioactive diagnostic material and products that may have been contaminated by same;
- Cytotoxic waste including any hazardous substance that is teratogenic, mutagenic or carcinogenic such as chemotherapy drugs; and
- General waste that may not have a chemical, biological, physical or radioactive hazard.
Scientific landfill
Is a kind of landfill that is a scientifically designed construction. The most important problem in ordinary landfill, is that the solid waste seeps into the underlying soil and water thus contaminating them. In scientific landfills a base layer of 90 metres of clay is constructed, thus the risk of waste seeping into the underground water and soil is eliminated. On top of the base layer a drainage layer made of soil, measuring 15 metres in length and a vegetative layer of 45 centimetres to minimise soil erosion. The presence of these layers ensures the safety of underground water and soil.
This method also helps as a degassing system, and also helps in the production of methane, as the layers soak most of the impurities in the disposed waste, methane is produces slowly compared to the ordinary landfills where methane is generated very fast. Vertical walls are also installed in scientific landfills, to help in extraction of methane regularly and the gas can then be used for electricity and heat generation purposes.
Composting
This is also another method of treating municipal solid waste, it’s a biological process where the organic portion of refuse is decomposed under controlled conditions, the presence of microbes help to metabolize the organic waste material and helps in reducing the volume to almost 50%. The stabilized product is called Compost or Humus. The compost resembles soil in texture and odour and can be used as soil conditioner or mulch. Compost is a mixture of organic matter, obtained has been digested of decomposed by microorganisms from leaves and manure. They are utilised to improve the soil structure and also provide nutrients.
Recycling and Reuse
It’s a recovery practise, where a collection of waste material is re-used for various purposes, the items used for recycling can be reprocessed into new products. Materials required for recycling are collected separately from the general waste using dedicated bins and also collection vehicles are sorted directly from the mixed waste streams, the owner need to separate the waste into different bins before it is collected for various purposes. The most common recycled products include aluminium like beverage cans, copper wires, aerosol cans, steel food old steel equipment’s or furnishings. Papers likes magazines, cartons, newspapers, corrugated boxes, bottles, jars etc. The recycling of complex material like computers are difficult to recycle as there need a lot of separation of parts and additional dismantling, the material used for recycling differs from every place. Every city or country have different recycling programs that handle a range of recycling materials. Eventually the resale value of the reprocessed product counts and the acceptance of these products matters.
Water Chemistry
Water is a chemical compound consisting of two hydrogen atoms and one Oxygen atom. The name water typically refers to the liquid state of the compound. The solid phase is called as ice and the gas phase is called as steam. Under specific conditions, water also forms a supercritical fluid. Water is the main compound found in living organisms. Approximately 62 percent of the human: body contains water. The word "water" comes from the Old English word water or from the Proto-Germanic water or German Wasser. All of which mean "water" or "wet." The boiling point of water is 99.98 degrees C (211.96 degrees F; 373.13 K).
There are several impurities in water
BIOLOGICAL IMPURITIES IN WATER
Biological impurities are caused due to the presence of microorganisms, they reproduce in water rapidly and contaminate the water, the organisms present in water include protozoans, algae, viruses, bacteria, microbes, pathogens and also parasites along with their eggs, contaminated water are one of the main causes of gastroenteritis in humans.
COLLOIDAL IMPURITIES IN WATER
Colloidal impurities present in water include the organic waste products, which occurs when suspended particles and elements like sand, organic matter and rocks that flow into rivers lakes streams and make the water impure and unable to drink it.
Sources of chemical impurities in water include:
- Atmospheric Gases that get mixed with rain water and torrential downpours.
- Impurities are added near streams, rivers and lakes, when animals and plants are decomposing near them.
- Waste water and sewage from industries.
- River water that contains higher levels of iron, calcium, magnesium, chloride and sodium.
- Anthrogenic contaminants, that are found in natural and drinking water, arise from organic compounds produced from industrial, domestic and agricultural waste.
- Other inorganic compounds that are formed from medical wastewater and other equipment systems.
Boiler Feed water
Boiler feedwater becomes an important part during boiler operations. The boiler feed water is fed into the steam drum, in the steam drum, the water turns into steam from the heat it receives, after the formation of steam it is carried to the main condenser, from the condenser it is pumped to the deaerated feed tank. From this tank it then goes back to the steam drum to complete its cycle. The feed water is never open to the atmosphere. This cycle is known as a closed system.
Since water impurities cause a lot of boiler problems, good water quality check is a must when water is taken for generating steam. The unique property of water is that, it absorbs more water than any other common inorganic substance. Water is known to expand around 1600times and forms steam at atmospheric pressure. The steam that is formed is capable of carrying large amounts of heat. All these properties of water make it an ideal raw material and also used in power generating processes.
The boiler water that is fed into drum must have a composition, such that the composition of the impurities must be of reasonable number., keeping the limits of the boiler design, if the limits are however exceeded the water must be pre-treated to remove the impurities. The impurities need not be completely removed in all cases, however, since chemical treatment inside the boiler can effectively and economically counteract them.
Sludge formation and Scale formation
- In boiler water evaporates continously and the concentration of salts left behind goes on increasing . After the saturation point they get precipitated.
- If the precipitate remains in boiler tube as loose and slimy matter is called sludge.
- If some of the precipitated matter adhers strongly and forms strong bad conducting layer on their inner side of boiler tube , then it is known as scale.
Sludge :-( formation of sludge )
- The loose slimy mass of salts precipitated in boiler water is the sludge.
- They are generally formed at cooler portion of boiler and they loosely deposit in the parts of boiler tube where flow rate is slow e.g vlves bends
- Sludges are easy to remove by using brushes detergent solutions blow down opreation e.t.c .
Scales formation
Scale is the hard and strongly adhered coating to the inner surface of boiler and it is a bad conductor of heat. It is the main source of boiler trouble.
Boiler corrosion
Corrosion in boilers occur in three ways, namely oxidation, electrolytic dissolution and by acid attack. The process of corrosion that occurs in boilers mainly an oxidational reduction reaction. When a metal dissoles in water , irrespective of water being acidic or alkaline, the metal is oxidized by hydrogen ion as the oxidising agent.therefore Iron will more readily dissolve in acid solutions that have high ion concentration. Boiler corrosion is an electrochemical process and is represented as
Fe----> Fe+ + 2 electrons
The reaction continues in the presence of oxygen as
4 electrons + O2+2H2O---------> 4OH
This indicates that iron corrodes in pure water,there is liberation of hydrogen ions and pH increases at the site of attack , until the water, metal and corrosion produts reach equlibrium.
The main cause for boiler corrosion occurs from dissolved gases, carbondioxide and oxygen found in feed water, the presence of oxygen and carbondioxide can cause corrosion of the feed water,boilers or even the condensate systems.Therefore the concentration of oxygen carbondioxide and dissolved gases in the feed water should be very low. This can be achieved by mechanical meanns followed by chemical treatmentto remove traces of these gases
Sea water is a potential source of acid as its salt contents especially magnesium chloride hydrolysis to yield acid, magnesium chloride reacts with water in the following manner
MgCl2 + 2H2O------> Mg(OH2) + 2HCl
As magnesium is insoluble it separates as sludge, leaving HCl in solution causing corrosion in boilers.
Sources of Water pollution
Some of the important sources of water pollution are: (i) Domestic effluents and sewage, (ii) Industrial effluents, (iii) Agricultural effluents, (iv) Radioactive wastes, (v) Thermal pollution, and (vi) Oil pollution.
Domestic Effluents and Sewage:
Man is the major consumer of water and uses water for a lot o domestic purposes such as cooking, bathing, cleaning etc, on an average per day 135 litres of water is used. Around 70% to 80% of this water is discharged and drained out, into municipal drains and in many cases flows into rivers, lakes etc.
This water is known as domestic waste water and, when other waste material such as paper, plastic, detergents, cloth, etc., is mixed in it; it becomes municipal waste or sewage.
The sewage and domestic waste water form the main sources of water pollution, this organic waste forms depletion of oxygen in water and upsets the natural balance of the aquatic ecosystem.
Municipal sewage is the main pollutant in water, before discharge of the water it is not treated, with the growing population, the waste water is also increasing in addition to large quantities of sewage, sewage consists of mainly decomposed organic matter and exert an oxygen demand on the receiving waters.
Industrial Effluents:
Industry forms the major range of waste products that are discharged into the water sources. Major contributors are the pulp and paper, chemicals, petrochemicals and refining, metal working, food processing, textile, distillery, etc. The wastes are usually heavy metals or organic synthetic compounds, these reach the water bodies either by direct discharge or by leaching from dumps
Agricultural water pollution is caused by fertilisers, insecticides and pesticides, farm animal wastes and sediments. In recent times, use of chemical fertilisers has shown a sharp increase. The green revolution in India is a reflection of the increased use of fertilisers. The chemicals used in fertilisers enter the groundwater by leaching and the surface waters by run-off.
Radioactive Wastes:
Radioactive elements like radium and uranium have a highly unstable atomic nuclei, the disintegration of these atoms in radiation emission is highly injurious. Many of the thermal and electric plants discharge hot effluents into the water bodies that causes thermal pollution in the water sources, warm water is undesirable as they do not have the same oxygen holding capacity as cold water.
As a result of this fishes like black bass, trout and walleyes etc that require a minimal amount of oxygen would rather move from the polluted waters or die in large numbers.
Oil Pollution:
The spread of oil in the sea has become a common feature nowadays. Oil is transported across oceans through tankers and either due to some accident or leakage oil spills onto the water and causes the degradation of aquatic and marine environment.
Biochemical Oxygen Demand:
The determination of the Biochemical Oxygen Demand or Biological Oxygen Demand (BOD) evaluates the amount of biodegradable organic material present in wastewater, effluent and polluted waters. The BOD test reflects the amount of dissolved oxygen (DO) consumed by bacteria while oxidizing these materials. Dissolved oxygen is essential for the life of aquatic fauna and flora, and the BOD test is a measure of the ecological impact that effluent water may have on the receiving body of water (river, lake, etc.). This test is often required in discharge permits, as it is a means of assessing the degree of water pollution.
Chemical Oxygen Demand: The chemical oxygen demand (COD) is a measure of water and wastewater quality. The COD test is often used to monitor water treatment plant efficiency. This test is based on the fact that a strong oxidizing agent, under acidic conditions, can fully oxidize almost any organic compound to carbon dioxide. The COD is the amount of oxygen consumed to chemically oxidize organic water contaminants to inorganic end products.
The COD is often measured using a strong oxidant (e.g., potassium dichromate, potassium iodate, potassium permanganate) under acidic conditions. A known excess amount of the oxidant is added to the sample. Once oxidation is complete, the concentration of organics in the sample is calculated by measuring the amount of oxidant remaining in the solution. This is usually done by titration, using an indicator solution. COD is expressed in mg/L, which indicates the mass of oxygen consumed per litter of solution.
COD is a second method of estimating how much oxygen would be depleted from a body of receiving water as a result of bacterial action. The COD test uses a strong chemical oxidizing agent (potassium dichromate or potassium permanganate) to chemically oxidize the organic material in the sample of wastewater under conditions of heat and strong acid. The COD test has the advantage of not being subject to interference from toxic materials, as well as requiring only two or three hours for test completion, as opposed to five days for the BOD test. It has the disadvantage of being completely artificial, but is nevertheless considered to yield a result that may be used as the basis upon which to calculate a reasonably accurate and reproducible estimate of the oxygen-demanding properties of a wastewater. The COD test is often used in conjunction with the BOD test to estimate the amount of non-biodegradable organic material in a wastewater. In the case of biodegradable organics, the COD is normally in the range of 1.3 to 1.5 times the BOD. When the result of a COD test is more than twice that of the BOD test, there is good reason to suspect that a significant portion of the organic material in the sample is not biodegradable by ordinary microorganisms. As a side note, it is important to be aware that the sample vial resulting from a COD test can contain leachable mercury above regulatory limits. If such is the case, the sample must be managed as a toxic hazardous waste.
- Determination of COD The method followed is dichromate method, water is oxidised in the presence of a strong oxidizing agent, potassium dichromate under acidic conditions. Additional potassium dichromate is added to ensure complete oxidation of the organic matter, after complete oxidation the excess amount of dichromate added is measured by titrating with ferrous ammonium sulphate, using ferroin as the indicator. The end point is ferroin changes from blue green to reddish brown, the amount of consumption of potassium dichromate is equal to the amount of organic matter present in the water sample.
3[CH2O] + 16H+ + 2Cr2O72- → 4Cr3+ + 3CO2 + 11H2O
Chemical oxygen demand calculation
COD in mg/L= 8000 (A-B) N / V
Where,
A is the volume of FAS used in the blank sample, in millilitres.
B is the volume of FAS in the original sample, in millilitres.
N is the normality of FAS solution.
V = millilitres of sample used for the test.
- Numerical problem
A 25 ml of sewage water sample was refluxed with 10 ml 0f 0.25 N K2Cr2 O7 solution. The untreated dichromate requires 6.5 ml of 0.1 N FAS.10 ml of dichromate solution and 25 ml distilled water, under the same condition as sample required 27 ml of 0.1 n FAS. Calculate the COD of sewage.
COD = V2 –V1 X N X 8 X 1000/V
V2 = volume of FAS for blank titration
V1 = volume of FAS for sample titration
V = volume of sample taken for test
N = Normality of FAS
COD = 27 –6.5 X 0.1X 8 X 1000/25
COD = 656 ppm
Sulphates (Gravimetry)
The amount of sulphate that is present is quantitatively determined as barium sulphate by gravimetric analysis.
In this method, an unknown sulphate sample is slightly acidified with concentrated hydrochloric acid, to this hot solution, a dilute solution of barium chloride is added. This results in the formation of a precipitate, that is filtered washed with deionised water and dried in the oven and weighed as Barium sulphate, the percentage of sulphate is calculated from the weight of barium sulphate.
The Gravimetry process gets its name by isolating the desired constituent in a form that can be weighed, in short it involves the change in the compound that contains the constituent into another constituent and then measuring the percentage of sulphate in the previous product.
Fluorides (Colorimetry)
Colorimetric methods have been developed to measure phenols, phosphorus, cobalt, ammonia, fluoride, and many other compounds in water. In order to measure each of these compounds the water sample is mixed with a reactant which interacts with the compound of interest. This reaction causes a colour change, with the intensity of colour dependent upon how much of the compound of interest was in the sample. The exact intensity of colour can be measured using a colorimeter.
For example, if ammonium molybdate and potassium tartrate are mixed with the sample these will combine with phosphorus, adding ascorbic acid to the sample will change this complex to a blue colour. In the case of measuring fluoride, the reagent (zirconium SPADNS) has a blue-ish colour to start out with. But when fluoride reacts with this reagent it bleaches the colour. So, the less blue colour in the final product, the more fluoride that is in the sample.
Primary method
In this method, the sewage flows through large tanks called as pre settling basins, or primary clarifiers, or primary sedimentation tanks, the tank are used to settle the sludge, while the oils and grease rise to the surface of the tank and are skimmed off. The primary tanks are provided with scrapers that are mechanically driven, that help to continually drive the sludge that is collected to a hopper that is present at the base of the tank, where it is later pumps to sludge treatment methods, the oil and grease is however recovered by saponification
Secondary treatment
In this method, the waste is substantially degraded, on the basis of the biological content of sewage present in human waste, food waste, soaps and detergents. Aerobic biological process is implemented by majority of the municipal plants. Microorganism like the bacteria and protozoa consume the biodegradable organic contaminants like fats organic carbon molecules.
Suspended-growth systems include activated sludge, in the activated sludge the biomass is mixed with the sewage and require smaller space than trickling filters, both require the same amount of water.
Tertiary treatment
Before the water is discharged to the environment (sea, river, ground, wet lands), the water is treated finally in the tertiary treatment, it is carried out mainly to improve the effluent quality of the water. Therefore, at the treatment plant more than one treatment process is carried out, the final process is always is disinfection.
Ion exchange technology is a proven method of producing high purity softened and demineralized water. It is used in most industries that require high purity water and to reclaim water from processes. The Ion exchange process involves the exchanging of contaminant ions for Na+ ions in a softening application and H+ and OH- ions in pure water application. Cations and anions can be removed by the cation and anion exchange resins. Resins containing –COOH, SO3H are capable for exchanging their H+ ions to cationic portion of minerals then it is called as cation exchanger while the resins containing –NH2, NHCH3 are capable for exchanging the anionic portion of the minerals then it is termed as anionic exchanger.
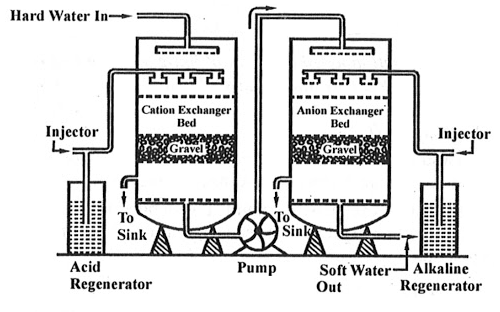
On supplying the hard water in first chamber which consists of Ca2+ or Mg2+ then the cation exchanger exchanges it with H+ hence the cation exchanger absorbs the Ca2+ ions the left water is free from cations are passed to another chamber by the help of pump this water consists of anions such as Cl or SO4 on sprinkle up of these water at anion exchanger bed then it exchanged the anions and hence release the demineralise water. The absorbed cation and anion are sinked out through the outlet present in chamber.
Reverse osmosis is a process where the water is separated from the salts in the source water by pressure-driven transport through a membrane. This process uses semi-permeable membrane and applied pressure to preferentially induce water permeation through the membrane while rejecting salts.
Desalination by RO requires the use of an osmotic membrane (i.e., one that allows water to pass through it at much higher rates than dissolved salts). The Osmotic membranes are present naturally in living organisms everywhere. The osmotic membrane is also called as semi permeable membrane, this membrane has the potential to allow some constituents to pass through them while the others are left behind.
In the process the dilute solution with a low concentration moves toward a high concentration through the semi permeable membrane.in the desalination of reverse osmosis process, a greater pressure than the osmotic pressure when applies to saline water will allow the fresh water to flow through the membrane while the solutes are held back, the higher the pressure applied above the osmotic pressure, higher will be the fresh water that will be transported across the membrane.
References:
1. O.G. Palanna, "Engineering Chemistry", Tata McGraw Hill Education Pvt. Ltd. New
Delhi, Fourth Reprint (2015- Edition).
2. R.V. Gadag & A. Nityananda Shetty., "Engineering Chemistry", I K International
Publishing House Private Ltd. New Delhi (2015- Edition).
3. "Wiley Engineering Chemistry", Wiley India Pvt. Ltd. New Delhi. Second Edition- 2013.
4. B. Jaiprakash, R. Venugopal, Sivakumaraiah and Pushpa Iyengar, Chemistry for Engineering Students, Subhash Publications, Bengaluru, (2015-Edition).