Unit 01
Question Bank
Q-1Enlist the WHO guidelines for drinking water.
(i) Drinking water must be supplied under continuous positive pressure.
(ii) Drinking water is unmodified except for the limited treatment of the water from a natural source.
(iii) It is common for drinking-water to be derived from a public water supply that may be a combination of more than one of the natural sources.
(iv) It is also common for public water supply organizations to conduct tests and guarantee that the drinking-water delivered is of drinking quality.
(v) Drinking-water quality is covered by the WHO drinking-water guidelines, standards from the International Organization for Standardization (ISO) and other regional and national agencies. Drinking-water should comply with the relevant regulations laid down by the competent authority.
Q-2Explain the hardness determination of sand by EDTA method.
Principle: This is a complex metric method. It is in the form of its sodium salt which yields the anion and this forms complex with Ca+2 and Mg+2 ions.
(Molecular Wt. - 372.24, Equivalent Wt. - 186.14 i.e., M=2N)
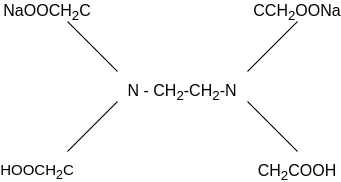
In order to determine the equivalence point (i.e., just completion of metal-EDTA complex formation) indicator Eriochrome Black-T (EBT) an alcoholic solution of blue dye is employed which forms an unstable wine red complex with Ca+2 and Mg+2 ions. The indicator is effective at about pH 10. When EBT is added to hard water, buffered to a pH of about 10 (employing NH4OH-NH4Cl buffer), a wine red unstable complex is formed. Thus,
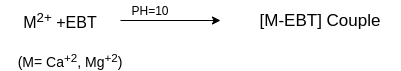
During the course of titration against EDTA solution, EDTA combines with M+2 (or Ca+2 or Mg+2) ions from stable complex M-EDTA and releasing free EBT, which instantaneously combines with M+2 ions still present in the solution, thereby wine red color is retained. Thus, titration
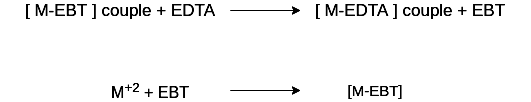
When nearly all M+2 (Ca+2 or Mg+2) ions have formed [M-EDTA] complex, then next drop of EDTA added drop wise displace the EBT indicator from [M-EBT] complex and wine red color changes to blue color (due to EBT). Thus at equivalence point

Steps involved:
1. Preparation of Standard Hard Water: Dissolve 1gm of pure dry CaCO3 in minimum quantity of dil. HCl and then evaporate the solution to dryness on water bath. Dissolve the residue n distilled water to make 1L solution. Each 1ml of this solution contains 1mg of CaCO3 hardness. 6
2. Standardization of EDTA solution: Rinse and fill the burette with EDTA solution. Pipette out 50ml of standard hard water in a conical flask. Add 10-15ml of buffer solution and 4 drops of indicator. Titrate with EDTA solution till wine red color changes to clear blue. Let the volume used be V1ml.
3. Titration of Unknown Hard Water: Titrate 50ml of water sample just in step5. Let the volume used be V2ml.
4. Titration of Permanent Hardness: Take 250ml of water sample in a large beaker. Boil till the volume is reduced to about 50ml (all the bicarbonates are decomposed into insoluble CaCO3+Mg(OH)2). Filter, wash the precipitate with distilled water collecting filtrate and washings in a 250 ml measuring flask. Finally make up the volume to 250ml with distilled water.
Q-3What are coagulation?
After all the large objects are removed from the original water source, various chemicals are added to a reaction tank to remove the bulk suspended solids and other various contaminants. This process starts off with an assortment of mixing reactors, typically one or two reactors that add specific chemicals to take out all the finer particles in the water by combining them into heavier particles that settle out. The most widely used coagulates are aluminum-based such as polyaluminum chloride.
Q-4Explain filtration.
The next step is generally running through some type of filtration to remove any suspended particles such as sediment, turbidity, and certain types of organic matter. It is often useful to do this early on in the process, as the removal of suspended solids upstream can help protect membranes and ion exchange resins from fouling later on in the pretreatment process. Depending on the type of filtration used, suspended particles can be removed down to under one micron.
Q-5What are breakpoint chlorination?
It involves addition of sufficient amount of chlorine to oxidize organic matter reducing substance and free ammonia in raw water by leaving behind free chlorine which possesses disinfecting action against disease - producing bacteria. The addition of chlorine at the dip or break is called “break point” chlorination.
Q-6Enlist the advantages of breakpoint chlorination.
(1) It oxides completely organic compounds, ammonia and other reducing compounds.
(2) It removes color, odor and taste of water.
(3) It removes completely all the disease causing bacteria/micro- organism
(4) It prevents the growth of any weeds in water.
Q-7Explain Deaeration.
At this point in the boiler feed water treatment process, any condensate being returned to the system will mix with the treated makeup water and enter the deaeration or degasification process. Any amount of gasses such as oxygen and carbon dioxide can be extremely corrosive to boiler equipment and piping when they attach to them, forming oxides and causing rust. Therefore, removing these gases to acceptable levels (nearly 100%) can be imperative to the service life and safety of the boiler system. There are several types of deaeration devices that come in a range of configurations depending on the manufacturer, but generally, we might use a tray- or spray-type deaerator for degasification or oxygen scavengers.
Q-8Explain Scale & sludge formation.
The water evaporates continuously and the dissolved salts concentration increases progressively. When their concentrations reach at saturation point, they are thrown out of water in precipitate form which get stick in inner walls of boiler. If the precipitation takes place in the form of loose or slimy precipitate it is called sludge. While if the precipitated matter forms a hard adhering coating on inner walls of boiler, then it is called as scale. Eg- MgCO3, MgCl2, MgSO4 etc.
Q-9Explain priming & foaming.
The duration at which boiler is producing steam rapidly, some particles of the condensed liquid are carried along with the steam. The process of wet steam formation is called priming. Priming is mainly caused by the presence of large amounts of dissolved solids, high steam velocities,sudden boiling etc. Whereas the continuous production of foam or bubbles in boilers which do not break easily are called as the foaming. This is caused due to the presence of substance like oils in water that reduce the surface tension in water.
Q-10Explain caustic embrittlement.
The use of high alkaline water in the boiler cause rust in the boiler which is called as Caustic Embrittelment. The presence of sodium carbonate plays a major role during the softening process.
Na2CO3 + H2O → NaOH + CO2
the caustic embrittelment is caused by using sodium phosphate as a softening agent instead of sodium carbonate.
Q-11Explain reverse osmosis.
Reverse osmosis (RO) and nanofiltration (NF) are often used down the line in the boiler feed water treatment system process so most of the harmful impurities that can foul and clog the RO/NF membranes have been removed. Similar processes of separation, they both force pressurized water through semipermeable membranes, trapping contaminants such as bacteria, salts, organics, silica, and hardness, while allowing concentrated, purified water through. Not always required in boiler feed water treatment, these filtration units are used mostly with high-pressure boilers where concentration of suspended and dissolved solids needs to be extremely low.
Q-12Explain lime soda process.
Surface water hardly exceed hardness level above 200 mg/1 and softening is not at all required in most of the cases, unless the water is being polluted by some effluent sources. In case of groundwater, hardness level of more than 1000 mg/1 are quite common. Since, soft water is corrosive, therefore public water supply are usually not softened below 30 to 50 mg/1. The most accepted and commonly used water softening methods are cat ion exchange and precipitation method. In order to obtain maximum profit, the factors to be considered are a good choice of a softening process, quality of the raw water, the cost of softening chemicals and the cost of disposing of waste streams.
Precipitation methods
The principle that follows the precipitation method is to bind calcium cations Ca and magnesium cations Mg , with ions of CO3 and OH . The precipitate CaC03 and Mg (OH)2 formed are removed from the water. Slake lime Ca(OH)2, Quick lime CaO , soda ash NaC03 and sodium hydroxide (caustic soda) NaOH, are reagents that are commonly used in water softening. Depending upon the quality of initial water, the following main precipitation methods are determined. a) Lime softening b) Lime - Soda softening c) Sodium Hydroxide softening Lime affects the carbonate hardness (alkalinity) and therefore can be used in order to decrease the carbonate hardness present in the initial water. This method however does not result in deep softening. Magnesium is removed from water if there is excess of OH” present. Water dissolved carbon dioxide is removed, total solids in the treated water diminishes and the total hardness in the lime treated water also reduces. But the pH increases to 10 or beyond. When lime is added to the hard water following reactions occurs, In the above reactions,
Lime Addition:-
Hardness Lime Precipitate
CO2 + Ca(OH)2 -- > CaCO3 + H2O Ca(HCO3)2 + Ca(OH)2 -- > 2CaCO3 + 2H2O Mg(HCO3)2 + Ca(OH)2 -- > CaCO3 + MgCO3 + 2H2O MgCO3 + Ca(OH)2 -- > CaCO3 + Mg(OH)2 CO2 the insoluble products do not contribute to the hardness, but It reacts with the lime, and thereby uses up some lime before the lime can start removing the hardness in water. Lime - Soda softening method is commonly practiced in most of the Public water supply. (Belan1984) The method is universal as water of almost any composition is treated with lime and soda. In this treatment, two reagents are used namely lime and soda ash. Lime as earlier discussed , decreases the carbonate hardness, (Mg2+) and removes C02 from the water.
Soda therefore reduces the non - carbonate hardness, mainly due to Ca2+, that shows after reaction with lime and the reaction occurs after the addition of soda ash is as follows.
Lime and Soda ash Addition:-
Lime Precipitate
MgSO4 + Ca(OH)2 Mg(OH)2 + CaSO4
Soda ash Precipitate
CaSO4 + Na2CO3 CaCO3 + Na2SO4