Unit 03
Question Bank
Q-1Explain the concept of electrode.
The electrode potential is defined as the Potential difference developed between the metal ions from metal to the solution or from solution to the metal. At equilibrium the potential difference remains constant. The electrode potential of a metal is defined as the direct measure of its tendency to get reduced is called reduction potential, its value is +x volts. Similarly the tendency of an electrode to lose electrons is a measure of its tendency to get oxidized is called oxidation potential, its value is –x volts.
Q-2Derive the expression of electrode potential.
Consider the following redox reaction
Mn+ + ne- ↔ M
For such a redox reversible reaction, the free energy change (∆ G) and its equilibrium constant (K) are related as;
∆ G = -RT ln k + RT ln [product]/[Reactant]
∆ G0 + RT ln [product]/[Reactant]………………..(i)
Where
∆ G 0 = standard free energy change.
The above equation is known as Van’t Hoff Isotherm.
The decrease in free energy in the reversible reaction will produce electrical energy i.e.
-∆ G = nEF and ∆ G 0 = -nE0F…………………………(ii)
Where
E = Electrode potential
E0 = Standard electrode potential
F = Faraday (96,500 coulombs)
Comparing equation 1 & 2
-nEF = -nE0F + RT ln [M]/[Mn+]
= -nE0F + Rt ln 1/ [Mn+]
Where, concentration of the metal is unity or
-nEF = -nE0F - RT ln [Mn+]
Dividing the equation by –nF
E= E0 + RT ln [Mn+]/nF
E= E0 + 2.303RT log [Mn+]/nF
E= E0 + 0.0591 log [Mn+]/n ……………(iii)
This equation-3 is known as “Nernst Equation” for electrode potential
Q-3What is Calomel electrode?
Calomel electrode is particularly very simple to construct, free from surface sensitivity and accurate to use even in a very normal laboratory.
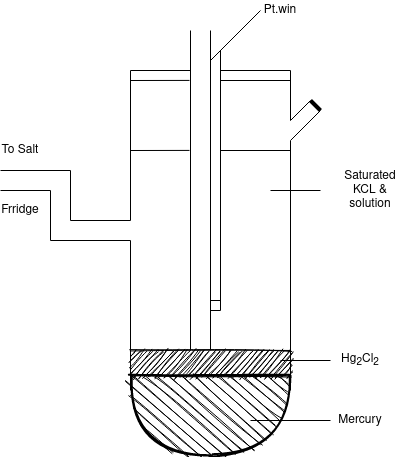
The calomel electrode consists of an inner glass tube and an outer jacket. In the inner glass tube a platinum wire is dipped into mercury which rests on a paste of mercurous chloride, Hg2Cl2 (commercially known as calomel) and mercury. This paste is in contact with KCl present in the outer jacket, through the glass frit plug fixed at the bottom of inner glass tube. The calomel electrode comes in contact with the experimental solution through a frit arranged to the outer jacket. The potential of this electrode depends on the concentration of KCl taken in the outer jacket.
Q-4Explain quinhydrone electrode with its advantages and limitations.
This is a redox electrode reversible to protons and often replaces the hydrogen electrode. Quinhydrone is a 1:1 molar mixture of quinone and hydroquinone. The electrode consists of a shiny platinum electrode dipped in an acid / base test solution, which is saturated with quinhydrone.
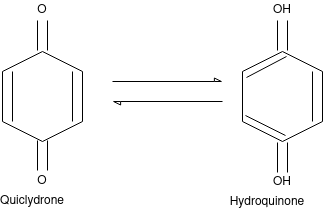
Advantages:
- The quinhydrone electrode is simple to set up and needs no removal of air.
- The reversibility equilibrium is achieved faster than hydrogen gas electrode thereby allowing a quicker measurement.
- PH values of solutions containing reducible substances like Cu2+, Cd2+, unsaturated acids, NO3 - , etc., and catalytic poisons can be measured using quin-hydrone electrode.
Limitations:
- The electrode cannot be used at pH values greater than 8.
This electrode also fails in the presence of strong oxidizing and reducing agents.
Q-5What are glass electrode?
Most often used pH electrodes are called glass electrodes and belong to the family of ISE. They are sensitive only to H+ ions. Typical glass electrode is made of glass tube engaged with small glass bubble sensitive to protons. Inside of the electrode is usually filled with buffered solution of chlorides in which silver wire covered with silver chloride is immersed.
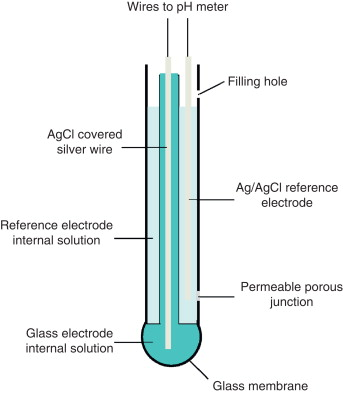
PH of internal solution varies- E.g.; it can be 1.0(0.1M HCl) or 7.0 Active part of the electrode is the glass bubble. While tube has strong and thick walls, bubble is made to be as thin as possible. Surface of the glass is protonated by both internal and external solution till equilibrium is achieved. Both sides of the glass are charged by the adsorbed protons, this charge is responsible for potential difference. This potential in turn is described by the Nernst equation and is directly proportional to the pH difference between solutions on both sides of the glass. The majority of pH electrodes available now a day are combination electrodes that have both glass H+ ion sensitive electrode and reference electrode compartments, conveniently placed in one housing.
Q-6Explain Lithium cell and its general uses.
It consists of lithium anode with solid electrolyte or liquid electrolyte and solid or liquid cathode. A thin protective insulating film is formed on lithium anode protecting the anode against corrosion as it is conductive to lithium ions but not electrons while water and alcohol never form such film.
Lithium iodide solid cathode cell consists of iodine PVP cathode with 3V voltage. It is highly stable and dependable and hence used in medical source for electronic flash guns of cameras.
Anode: Graphite, Carbon compound.
Cathode: Oxide of Lithium
Uses:
Used in Laptops, cellular phones, electronic vehicles.
Q-7Explain lithium ion battery with its charge chemistry.
Lead storage battery is the most common device used to store energy in the portable form. This is also called as lead acid battery. Although the batteries are reliable, which contain acidic material inside that required a proper disposal method after its complete use. These batteries have moderate power density and good time. The battery consists of lead grids on its electrodes. The anodic grid opening is filled with spongy lead while the cathodic grid consists of lead oxide (PbO2).
Charge Chemistry of the battery:
Charge batteries are those batteries which can be recharged after single use. In this type of battery each plate contain negative as well as the positive end. The negative plate is of lead while the positive plate is made up of lead oxide in an electrolyte of approx. 4.0M sulphuric acid.
Negative plate reaction:
PbSO4(s) + H+(aq) + 2e– → Pb(s) + HSO4–(aq)
Positive plate reaction:
PbSO4(s) + 2H2O(l) → PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e–
Combining these two reactions, the overall reaction is the reverse of the discharge reaction:
2PbSO4(s) + 2H2O(l) → Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq)
Discharge Chemistry of the Battery:
The positive and negative plate of the batteries becomes lead sulphate. Due to the loss of sulfuric acid from electrolytes it becomes the water.
Negative plate reaction:
Pb(s) + HSO4–(aq) → PbSO4(s) + H+(aq) + 2e–
Positive plate reaction:
PbO2(s) + HSO4–(aq) + 3H+(aq) + 2e– → PbSO4(s) + 2H2O(l)
Combining these two reactions, one can determine the overall reaction:
Pb(s) + PbO2(s) + 2H+(aq) + 2HSO4–(aq) → 2PbSO4(s) + 2H2O(l)
Q-8Explain electrochemical series and its applications.
The deterioration of materials by chemical process is called as corrosion. In electrochemical corrosion M → M+ + e- is facilitated by the presence of suitable electron acceptor and some time it is also called as depolarizer. Corrosion can also be viewed as the spontaneous return of metals to their ores, the abandoned amount of energy used that were consumed in the mining, refining into useful objects is dissipated by variety of different routes.
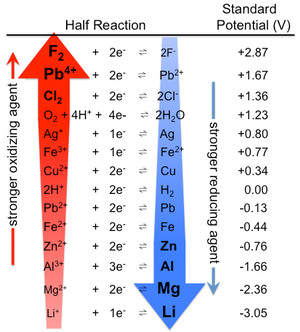
Corrosion Cells and reactions:
The occurrence of oxidation and reduction steps in corrosion results in separation of metal’s location. This can be possible due to the conductive property of metal, so that electrons can flow through metal form anodic to cathodic regions. The water presence plays a major role on transportation.
Fe → Fe2+ + 2e-
The metal is under pressure or is isolated from the air. The metal ions dissolve in the moisture film and e- migrates to another location.
The anodic process is
Fe(s) → Fe2+ (aq) + 2e-
While cathodic steps involve the reduction of oxygen gas
O2 + 2H2O(aq) + 4e- → 4OH-
Or the proton reduction
H+ + e- → ½ H2(g)
Or the reduction of metal ion
M2+ + 2e- → M(s)
Since both the cathodic and anodic steps must take place for corrosion to occur, prevention of either one will stop corrosion. The most obvious strategy is to stop both processes by coating the object with a paint or other protective coating. Even if this is done, there are likely to be places where the coating is broken or does not penetrate, particularly if there are holes or screw threads. A more sophisticated approach is to apply a slight negative charge to the metal, thus making it more difficult for the reaction to take place:
M⟶M2++2e−.
Applications:
- The thermal stability of the metal oxide depends on its electropositive nature. As the electro positivity decreases from top to bottom, the thermal stability of the oxide also decreases from top to bottom.
- A more electropositive metal can displace a less electropositive metal from its salt's solution.
The metal which can provide electrons to ions present in dilute acids for reduction, evolve hydrogen from dilute acids.
Q-9What are fuel cell?
Fuel cell is the electrochemical cell that helps in the conversion of chemical energy of a fuel and oxidizing agent into electricity by the help of redox reaction. Fuel cell has to be supplied by an external source of fuel and an oxidant. The hydrogen or any other fuel is oxidized electrochemically inside the fuel cell to produce a potential difference i.e. a voltage capable of producing a working current. The overall chemical reaction in a hydrogen fuel electrochemical cell involves the oxidation of hydrogen by oxygen to produce only water. Hydrogen fuel cells offer an alternative to rechargeable cells and batteries. A fuel cell will produce a potential difference and a workable electric current until one of the reactants is used up.
Hydrogen gas can be used as fuel:
2H2(g)+ O2(g) 2H2O(l)
It is an exothermic reaction, releasing lots of heat energy when burnt, remember the 'squeaky pop' lit splint test for hydrogen. The hydrogen - oxygen fuel cell is non-polluting, since only water is produced.
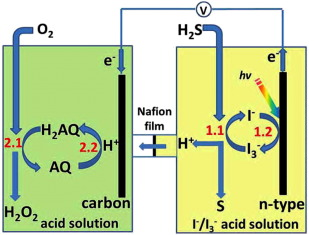
A hydrogen–oxygen fuel cell is a non–polluting clean fuel. Fuel cells do not produce pollutants like carbon monoxide, sulfur dioxide and nitrogen oxides. Cars powered by fuel cells would be quite an environmental advantage in cities, where electric cars are already beginning to be significantly used in developed countries. Fuel cells could replace larger batteries which are not easily recycled and contain highly toxic metal compounds. It would be an ideal fuel on this basis e.g. For motor vehicles, but that's not the only factor to consider. It would be ideal if the hydrogen fuel could be manufactured by electrolysis of water e.g. Using solar cells.
Q-10Explain electrochemical corrosion.
Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by the elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
(ii) when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
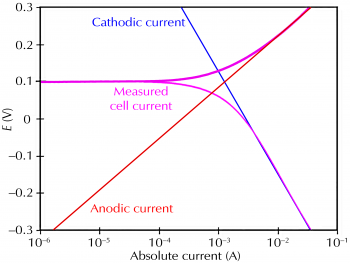
Corrosion process showing the anodic and cathodic component of current
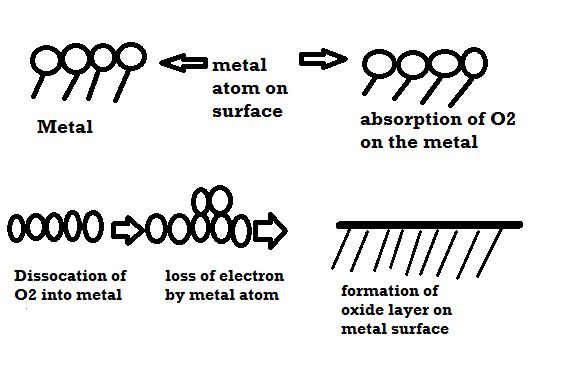
Mechanism of dry corrosion due to O2 gas there are 4 types: -
- Absorption of oxygen molecules on the metal surface
- Dissociation of oxygen atom into metal atom
- Loss of e- by metal atom
- Formation of oxide layer on the metal surface.
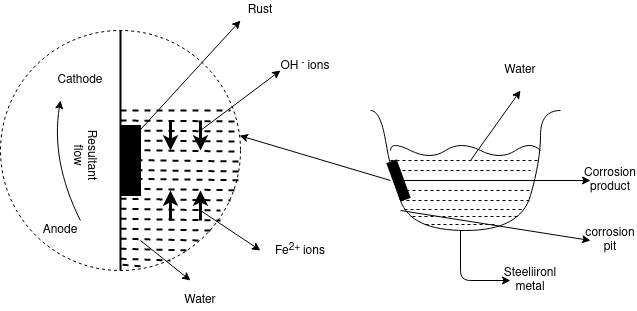
Q-11Explain differential aeration corrosion.
The uneven supply of oxygen to the same metal component leads to the formation of oxygen concentration cells that are called as the differential aeration theory of corrosion. It is the type of electrochemical corrosion that affects the metals such as steel and iron. The less oxygenated part behaves anodic while the more oxygenated part cathodic. Since cathodic reactions involve consumption of oxygen, the more oxygenated part behaves cathodic and less oxygenated pan behaves anodic. The reaction occurs because oppositely charged electrons flow between the smaller anode and larger cathode. Positively charged cations meeting negatively charged anions forming corrosion product and a resulting pit in the metal, otherwise known as pitting corrosion. In a gutter, pipe, tank or similar the anode is just below the waterline. This is where the oxidation occurs, corrosion product forms and a pit develops weakening the metal.
Q-12Explain electroplating Principle:
Electroplating is a method in which coating metal is coated on the base metal on the basis of electrolysis principle.
Processes:
- The article to be electroplated is cleaned well.
- There is a non-conducting tank which containing coating metal salts.
- The article is connected to the negative terminal of DC source which act as a cathode.
- Anode is a coating metal.
- After adjusting suitable PH and density on the base metal, then this method is started.
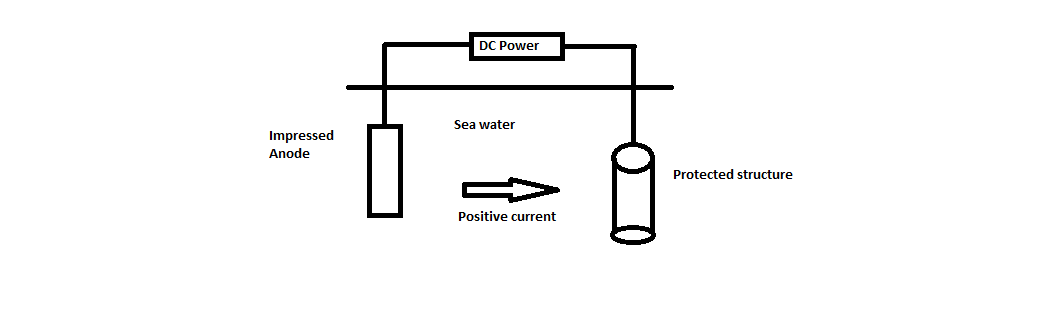
Metal ions in a solution migrate towards the article and get deposit on the base metal in the form of coating layer.
- Cr + 3e- - Cr (if cr plating)
- Ag+ + Ag - Ag (if Ag plating)
- Ni + + 2e- - Ni (if Ni plating)
At anode (Coating metal)
- Cr - Cr + + 3e –
- Ag - Ag + + e-
- Ni - Ni + + 2e-
Advantages: -
- Electroplating can be done on the article of any shape
- This is strong coating
Application: -
- Corrosion protection method
- Decoration
- It is also applicable on the non – metallic surface
- Electroplating is done on the many parts of machines.
- Coating metals: -Cu,Ni,Cr, Ag, etc.
- Base metals: - Fe (steel)non-metallic surface like glass.