Unit -3
Fuels
1.Differentiate between Enantiomers and Diastereomers?
The key difference between diastereomers and enantiomers is that diastereomers of a molecule are not enantiomers are mirror images of each other mirror images of each other while.
Enantiomers contain chiral centers that are non-superimposable & mirror images. They only come in pairs!
Diastereomers contain chiral centers which are non-superimposable but are NOT mirror images. There can be more than 2 depending on the number of stereocenters.
2. Explain Knocking and Anti-knocking?
Knocking, this usually occurs in an internal combustion engine, where sharp sounds occurs due to the premature combustion from the part of the compressed air-fuel mixture present in the cylinder. When an engine functions smoothly in the combustion chamber, the charge burns with the flame front smoothly progressing from the point of ignition. In some cases, depending upon the composition of the fuel, at high compression ratios, some of the charge may ignite spontaneously ahead of the flame front resulting in burning in an uncontrolled manner that produces pressure waves of high frequency. The waves are responsible for forcing parts of the engine to vibrate and produce audible sound.
The effects of Knocking can cause overheating of the spark-plug points, erosion of the combustion chamber surface, and rough, inefficient operation.
An antiknock agent is a gasoline additive that is used to reduce knocking that occurs in engines, the fuels octane number is increased by increasing the temperature and pressure at which auto-ignition occurs. As the gasoline engine developed, gasoline and the engine were harmonized to attain the best possible matching of characteristics. One of the important characteristics of gasoline is it is volatile in nature and antiknock quality, Volatility is a measure of the ease of vaporization of gasoline, Gasoline also helps to start engines in cold weather and to avoid vapour lock in hot weather conditions.
3. Differentiate between Proximate and Ultimate Analysis?
The key difference between proximate and ultimate analysis of coal is that proximate analysis is the technique used to analyse the moisture content, ash content and fixed carbon of coal whereas ultimate analysis is the technique used to analyse the chemical composition of coal.
The technique of proximate analysis involves the determination of the different compounds present in a mixture. Ultimate analysis, on the other hand, involves the determination of the number and types of different chemical elements present in a particular compound. Therefore, these two techniques are related to each other.
Proximate analysis of coal is the process of determining the presence of different compounds and their amounts in coal. The technique of proximate analysis was developed by Henneberg and Stohmann (German scientists) in 1860. This analysis technique involves the partitioning of compounds into different categories depending on the chemical properties of these compounds. Mainly, there are six categories of compounds as moisture, ash, crude protein, crude lipid, crude fibre, and nitrogen-free extracts. In the process of proximate analysis of coal, the moisture content of coal, ash content of coal and the fixed carbon content of coal are determined.
The ultimate analysis of coal is the process of determining different chemical elements present in coal. This technique allows us to get more comprehensive results compared to the proximate analysis process.
In this analysis technique, we test moisture, ash, carbon, hydrogen, nitrogen, sulphur and oxygen content of the sample to determine the elemental composition of the coal sample. Therefore, each and every chemical element in the sample is analysed through chemical routes and then we can express the contents as percentages with respect to the total mass of the sample. Mostly, this analysis technique is useful in the coal and coke industry.
4. What are Octane and Cetane numbers?
Octane Number and Cetane Number are the standards to measure the tendency of fuel to ignite spontaneously. The performance of gasoline is measured by the Octane number on the other hand the cetane number measures the performance of diesel. The reason why petrol can’t be used in diesel engine and diesel can’t be used in a petrol engine is that when the fuel that has high octane number will have low cetane number and high cetane number fuel has low octane number.
As per the Standard operating conditions the Octane number of a fuel defines the percentage of Iso-butene present in a mixture of Iso-octane and heptane. When used in a gasoline engine the Octane rating signifies the ability to resist auto ignition. As air and fuel are compressed together, gasoline tends to ignite a spark at the end of compression by a spark plug. If gasoline with low octane number is used it creates problems during ignition and tends to adapt to auto combustion easily due to excess of heat and pressure effects on the other hand, fuels that have high octane value takes more time to burn but provides maximum efficiency to the gasoline engine.
Cetane is a type of chemical compound known as a Hexadecane. Cetane number is opposite to octane number, and measures how quickly the engine burns inside a compressed engine. Cetane compounds tend to ignite spontaneously under compression, therefore they are assigned as cetane number of hundred. Cetane number of a fuel can be defined as the percentage volume of n-hexadecane in the mixture of n-hexadecane and 1-methylnaphthalene which is responsible to provide ignition delay period.
5. What is optical activity?
The optical activity of a substance, is the tendency of a substance to rotate along the plane of polarization of a beam of light that is passed through it. (In a plane-polarized light, the vibrations of the electric field are confined to a single plane.) The intensity of optical activity of a substance is expressed in terms of a quantity, called specific rotation, Optical activity was first observed in quartz crystals in 1811 by a French physicist, francois Arago. Another French physicist, Baptiste, found in 1815 that liquid solutions of tartaric acid or of sugar are optically active, as are liquid or vaporous turpentine. Louis Pasteur was the first to recognize that optical activity arises from the dissymmetric arrangement of atoms in the crystalline structures or in individual molecules of certain compounds.
6. Explain Conformational Isomerism.?
Conformational isomers are those isomers where the relative positions of few atoms differ in the molecule in the three-dimensional space because of the rotation about sigma bonds.
7. What are substitution and elimination reactions?
The key difference between elimination and substitution reaction can be best explained by using their mechanism. In elimination reaction, rearrangement of previous bonds occurs after the reaction, whereas substitution reaction replaces a leaving group with a nucleophile
It is a kind of chemical (substitution) wherein the replacement of one functional group in a chemical is compound by another. This reaction is also referred to as ‘single replacement reaction’ or ‘single displacement reaction’. This reaction is of utmost significance in organic chemistry. It is divided into two categories: Nucleophilic and Electrophilic.
It is a kind of organic reaction (elimination reaction) wherein two substituents are eliminated from a molecule in one or two-step mechanisms. Most of the elimination reactions result in the loss of at least one hydrogen atom and form double bond. The unsaturation of the molecule increases because of this factor
8. What are nucleophiles? Give an Example.
A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can be nucleophiles. This pair of electrons is called lone pair. Examples of nucleophiles are anions such as Cl −, or a compound with a lone pair of electrons such as NH 3 (ammonia)
9. Explain the Calorific Value?
Calorimeter, is a device that measures the heat developed when a chemical electrical or chemical reaction taking place, the device is also used to calculate the capacity of heat of materials.
The bomb calorimeter, consists of an enclosure that is surrounded by a liquid; however, the reaction occurs in the enclosure, the surrounding liquid may be water as it absorbs the heat produced in the reaction and thereby increases the temperature. If the weight and heat characteristics of the container is known, and the rise in temperature is measured, the total amount of heat can be calculated.
The design of a typical bomb calorimeter is shown below. The bomb is a steel reaction vessel, where the material to be analysed is deposited. The steel bomb vessel is placed inside a bucket that is filled with water, the temperature of the bucket is kept constant by using a stirrer and a heater. The variation in temperature is observed on a thermometer fitted with a magnifying glass, and the accurate reading are recorded. The presence of slots at the top of the steel bomb that allows the ignition wires and oxygen supply to enter the vessel as both these factors are critical for the reaction. Heat losses are minimized by inserting an air space between the bucket and an exterior insulating jacket. When an electric current pass through the ignition coil, a combustion reaction occurs. The heat released from the sample is largely absorbed by the water, which results in an increase in temperature. Bomb calorimeters have been developed to the point that heats of combustion of organic materials can be measured with results reproducible within 0.01 percent.
10.Explain the Diel Alder reaction.?
The Diels-Alder reaction is a conjugate addition reaction of a conjugated diene to an alkene (the dienophile) to produce a cyclohexene.
- The simplest example is the reaction of 1,3-butadiene with ethene to form cyclohexene:
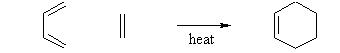
- The analogous reaction of 1,3-butadiene with ethyne to form 1,4-cyclohexadiene is also known:

- Since the reaction forms a cyclic product, via a cyclic transition state, it can also be described as a "cycloaddition".
- The reaction is a concerted process:

Due to the high degree of regio- and stereoselectivity (due to the concerted mechanism), the Diels-Alder reaction is a very powerful reaction and is popularly used in synthetic organic chemistry.