Unit - 7
Organic reactions and synthesis of a drug module
Q1) Explain the Intermediate process with an example.
A1) An intermediate is a process which appears in the mechanism of a reaction, but not in the overall balanced equation. An intermediate is always formed in an early step in the mechanism and consumed in a later step.
For e.g. One reaction that describes a reaction mechanism is the chemical reaction between nitrogen monoxide and oxygen to form nitrogen dioxide:
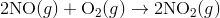
It seems as though this reaction occurs due to the collision between two NO molecules with one O 2 molecule. However, careful analysis of the reaction has detected the presence of N 2 O 2 during the reaction. A proposed mechanism for the reaction consists of two elementary steps:
Step 1: 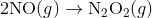
Step 2: 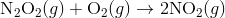
In the first step, two molecules of NO collide to form a molecule of N 2 O 2 . however, in the second step, that molecule of N 2 O 2 collides with a molecule of O 2 to produce two molecules of NO 2. The overall chemical reaction is the sum of the two elementary steps:
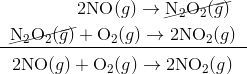
The N 2 O 2 molecule is not part of the overall reaction. It was first produced in the elementary step; the N 2 O 2 molecule then reacts in the second elementary step.
Q2) What happens when sugar is broken down in our body, name the reaction that takes place?
A2) The best example that happens within our bodies, is a sequence of oxidation-reduction reactions that occur to burn sugars, such as glucose (C6H12O6) and the fatty acids in the fats that we eat.
C6H12O6(aq) + 6 O2(g)  6 CO2(g) + 6 H2O(l)
6 CO2(g) + 6 H2O(l)
CH3(CH2)16CO2H(aq) + 26 O2(g)  18 CO2(g) + 18 H2O(l)
18 CO2(g) + 18 H2O(l)
These reactions can also be used as a source of energy,
Q3) Why do cars tend to rust easily? Give reasons
A3) When iron is heated, it reacts with oxygen to form a mixture of iron (II) and iron (III) oxides.
2 Fe(s) + O2(g)  2 FeO(s)
2 FeO(s)
2 Fe(s) + 3 O2(g) 2 Fe2O3(s)
Molten iron even reacts  with water to form an aqueous solution of Fe2+ ions and H2 gas.
with water to form an aqueous solution of Fe2+ ions and H2 gas.
Fe(l) + 2 H2O(l)  Fe2+(aq) + 2 OH-(aq) + H2(g)
Fe2+(aq) + 2 OH-(aq) + H2(g)
At room temperature, however, all three of these reactions are so slow they can be easily ignored.
Iron can only corrode at room temperature in the presence of both water and oxygen. In the sequence of this reaction, the iron gets oxidized to give a hydrated form of iron (II) oxide.
2 Fe(s) + O2(aq) + 2 H2O(l)  2 FeO H2O(s)
2 FeO H2O(s)
Because iron (II) oxide has the same empirical formula as Fe(OH)2, usually iron(II),is mistaken and also called as ferrous hydroxide. The FeO H2O formed in this reaction is further oxidized by O2 dissolved in water to give a hydrated form of iron (III), or ferric oxide.
4 FeO H2O(s) + O2(aq) + 2 H2O(l)  2 Fe2O3 3 H2O(s)
2 Fe2O3 3 H2O(s)
In a further complicated reaction, FeO H2O formed at the metal surface combines with Fe2O3 3 H2O to give a hydrated form of magnetic iron oxide (Fe3O4) shown in the reaction below.
FeO H2O(s) + Fe2O3  3 H2O(s) Fe3O4 n H2O(s)
3 H2O(s) Fe3O4 n H2O(s)
As these reactions only can occur in the presence of both water and oxygen, cars tend to rust easily wherever water collects
Q4) Explain the Diel Alder reaction.
A4) The Diels-Alder reaction is a conjugate addition reaction of
a conjugated diene to an alkene (the dienophile) to produce a cyclohexene.
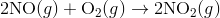
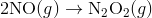
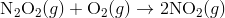
Due to the high degree of regio- and stereoselectivity (due to the concerted mechanism), the Diels-Alder reaction is a very powerful reaction and is popularly used in synthetic organic chemistry.
Q5) What are nucleophiles? Give an Example.
A5) A nucleophile is a species that donates an electron-pair to an electrophile to form a chemical bond in a reaction. All molecules or ions with a free pair of electrons can be nucleophiles. This pair of electrons is called lone pair. Examples of nucleophiles are anions such as Cl −, or a compound with a lone pair of electrons such as NH 3 (ammonia)
Q6) What are substitution and elimination reactions?
A6) The key difference between elimination and substitution reaction can be best explained by using their mechanism. In elimination reaction, rearrangement of previous bonds occurs after the reaction, whereas substitution reaction replaces a leaving group with a nucleophile
It is a kind of chemical (substitution) wherein the replacement of one functional group in a chemical is compound by another. This reaction is also referred to as ‘single replacement reaction’ or ‘single displacement reaction’. This reaction is of utmost significance in organic chemistry. It is divided into two categories: Nucleophilic and Electrophilic.
It is a kind of organic reaction (elimination reaction) wherein two substituents are eliminated from a molecule in one or two-step mechanisms. Most of the elimination reactions result in the loss of at least one hydrogen atom and form double bond. The unsaturation of the molecule increases because of this factor
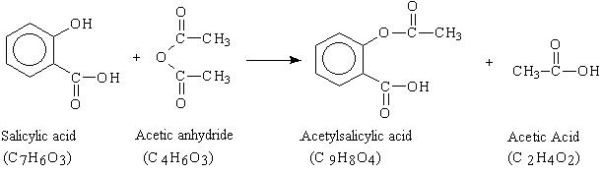
Q7) Who was the first person to synthesize aspirin and how?
A7) Aspirin (acetylsalicylic acid) is a synthetic organic derived from salicylic acid. Felix Hoffman a German chemist, is credited with being the first to synthesize aspirin in 1897. Hoffman's father had severe arthritis he used salicylic acid to relieve pain but could not tolerate salicylic acid. The name given for Hoffman's new compound was A-spirin. Apparently, this name comes from acetylation (A-), together with Spirin that is part of the name given for Meadow-sweet (Spiraea ulmaria), a plant rich in salicylates.
Friedrich Bayer, the employer of Hoffman, patented the name and began marketing the product in 1899. The product was a huge success and sales grew rapidly. Bayer's company, which he set up by himself, is reckoned to have been the first pharmaceutical company, and the production of aspirin is accepted to have laid the foundation of the modern pharmaceutical industry.
In this experiment aspirin (acetylsalicylic acid, C9H8O4) is synthesized and purified, and the percent yield is determined. The purity of the product formed is confirmed by measuring its melting point range and qualitative analysis.
Q8) Explain the formation of Aspirin with a chemical reaction?
A8) The reaction that is used for the synthesis is shown below. In this reaction, an excess of acetic anhydride (C4H6O3) is added to a measured mass of salicylic acid (C7H6O3) in the presence of a catalyst, sulfuric acid (H2SO4). The mixture is heated to form the acetylsalicylic acid (C9H8O4) and acetic acid (C2H4O2). After the reaction takes place, water is added to destroy the excess acetic anhydride and cause the product to crystallize. The aspirin is then collected, purified by recrystallization, and its melting temperature measured.