Module 04
Use of free energy in chemical Equilibria
Q-1 Explain entropy.
Answer:
Entropy is used to describe the behavior of a system in terms of thermodynamic properties such as temperature, pressure, entropy, and heat capacity. This thermodynamic description took into consideration the state of equilibrium of the systems.
Meanwhile, the statistical definition which was developed at a later stage focused on the thermodynamic properties which were defined in terms of the statistics of the molecular motions of a system. Entropy is a measure of the molecular disorder.
Q-2 Explain free energy in thermodynamic.
Answer:
In thermodynamics, energy-like property or state function of a system in thermodynamic equilibrium. Free energy has the dimensions of energy, and its value is determined by the state of the system and not by its history. Free energy is used to determine how systems change and how much work they can produce. It is expressed in two forms: the Helmholtz free energy F, sometimes called the work function, and the Gibbs free energy G. If U is the internal energy of a system, PV the pressure-volume product, and TS the temperature-entropy product (T being the temperature above absolute zero), then F = U − TS and G = U + PV − TS. The latter equation can also be written in the form G = H – TS, where H = U + PV is the enthalpy. Free energy is an extensive property, meaning that its magnitude depends on the amount of a substance in a given thermodynamic state.
Q-3Explain the relation between free energy and emf.
Answer:
When a cell produces a current, the current can be used to do work - to run a motor, for instance. Thermodynamic principles can be employed to derive a relation between electrical energy and the maximum amount of work, Wmax, obtainable from the cell. The maximum amount of work obtainable from the cell is the product of charge flowing per mole and maximum potential difference, E, through which the charge is transferred.
Wmax = - n FE
Where,
n is the number of moles of electrons transferred and is equal to the valence of the ion participating in the cell reaction.
F stands for Faraday and is equal to 96,495 coulombs and E is the emf of the cell.
According to thermodynamics, the maximum work that can be derived from a chemical reaction is equal to the free energy (DG) for the reaction,
Wmax = DG
Therefore, from (1) and (2),
DG = - n FE
Thus only when E has a positive value, DG value will be negative and the cell reaction will be spontaneous and the e.m.f. Of the cell can be measured. Here E refers to the Ecell.
Thus, the electrical energy supplied by the cell is (nFE) equal to the free energy decrease (- DG) of the cell reaction occurring in the cell.
Q-4Determine the standard emf of the cell and standard free energy change of the cell reaction.
Answer:
Zn, Zn2+ || Ni2+, Ni.
The standard reduction potentials of Zn2+, Zn and Ni2+, Ni half cells are - 0.76 V and - 0.25 V respectively.
Eocell = EoR - EoL = - 0.25 - (- 0.76)
= + 0.51 V Eocell is + ve. \ DGo = - ve.
DGo = - n F Eocell = 2 electrons
DGo = -2 * 96495 * 0.51
= -97460 Joules
= - 97.46
Q-5Derive the Nernst equation.
Answer:
Gibbs free energy: Gibbs free energy of the system is the difference of enthalpy of the system with the product of temperature times the entropy of the system.
G=H-TS
Gibbs free energy of the system is defined in term of the thermodynamics which are state in function. Any change in the Gibbs Free Energy System is directly proportional to the difference of change in the enthalpy of the system with the products of temperature times the entropy of the system.


 G= H- (TS)
G= H- (TS)
While at constant temperature this reaction transform into:


 G= H-T S
G= H-T S
The Nernst Equation is derived from the Gibbs free energy under standard conditions.
E*=E*reduction-E*oxidation………..(i)
 G=-nFE………..(ii)
G=-nFE………..(ii)
Where,
n=no. Of transferred electrons in the reaction
F= Faraday constant
E=Potential Difference.
While when we see in the standard condition then, equation (ii) becomes
 G*=-nFE*………….(iii)
G*=-nFE*………….(iii)
Hence,
Reaction is Spontaneous when E* is positive while non- spontaneous in vice-versa.

 G= G*+RT lnQ………….(iv)
G= G*+RT lnQ………….(iv)

 Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
Now, Substituting G=−nFE and G*=−nFE* into Equation 4, we have:
−nFE=−nFEo+RTlnQ…………….(v)
On dividing both sides of the Equation above by −nF,
E=E*−RTnFlnQ(6)……….(vi)
Equation (vi) in the form of log10:
E=E*−2.303RT/nF log10Q…….(vii)
At standard temperature T = 298 K, the 2.303RT/F term equals 0.0592 V and Equation
(vii) can be rewritten:
E=E*−0.0592V/n log10Q……..(viii)

 The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
The equation (viii) clearly indicates that electric potential of cell depends on reaction quotient of reaction. The product formation leads to the increase in the concentration of the products. This tends to decrease the the potential of the cell until it reaches at the stage of equilibrium where, G=0 and G=-nFE Q=K so E=0
Then on substituting the these values to Nernst Equation we get,
0=E*-RT/nF In K…….(ix)
At room temperature it becomes;
0=E*-0.0592V/n Log10K
LogK=nE*/0.0592V
The above equation clearly indicates the equilibrium constant K is proportional to the standard potential.
Q-6What are corrosion?
Answer:
The destruction of metal by chemical or electrochemical attack of environment this process starting at the surface of metal known as corrosion. Corrosion is the two step process that requires three things i.e., a metallic surface, an electrolyte and oxygen. In the process of corrosion a metal atom at surface dissolve into an aqueous solution leaving the metal with excess negatively charged ions. These resultant ions are removed by a suitable electron acceptor. Corrosion can be thought of as the spontaneous return of metals to their ores through the process of oxidation.
Q-7Explain the corrosion process showing cathodic current and anodic current.
Answer:
Corrosion occurs due to the electrochemical process of oxidation and reduction process. In the corroding solution electrons are released by the metal and that is gained by the elements in the corroding solution. The release of electron from metal is called as the oxidation while vice-versa that is gain of electron by elements is reduction. The regular electron flow in the corrosion reaction can be measured and controlled electronically. This is why controlled electrochemical experimental methods are used to characterize the corrosion properties of metal.
For example,
(i) a thin film of moisture on a metal surface forms the electrolyte for atmospheric corrosion.
(ii) when wet concrete is the electrolyte for reinforcing rod corrosion in bridges. Although most corrosion takes place in water, corrosion in non-aqueous systems is not unknown.
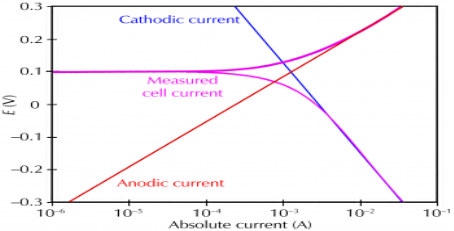
Corrosion process showing the anodic and cathodic component of current
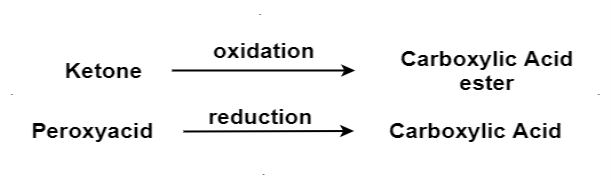
Q-8Explain oxidation process and its mechanism.
Answer:
The oxidation of ketone to a carboxylic acid ester by the help of peroxy acid as the oxidizing agent.
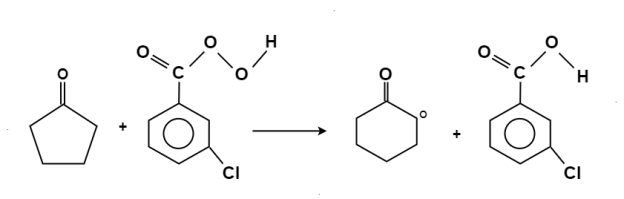
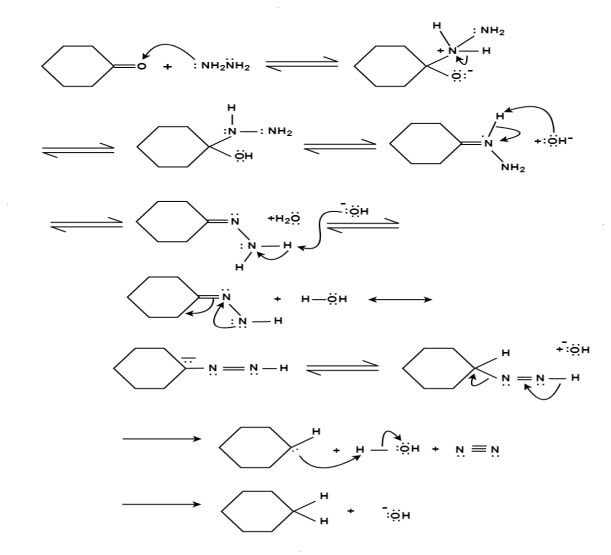
Mechanism:
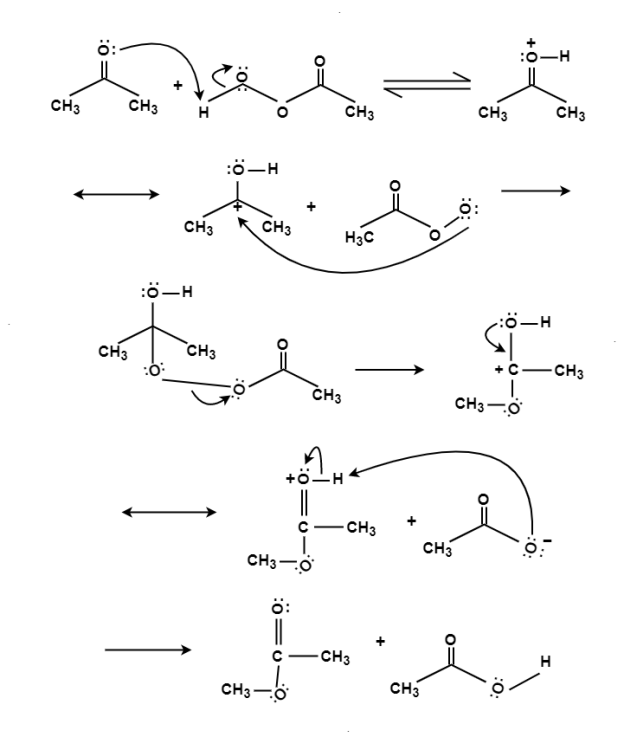
Q-9 Explain reduction reaction and its mechanism.
Answer:
The aldehydes and ketones react with hydrazine in basic medium, which reduces the aldehyde or the ketone to a hydrocarbon, is called Wolff-Kishner reduction.
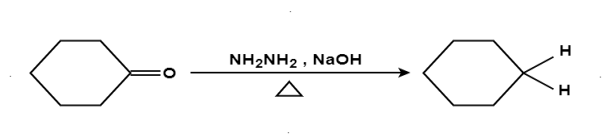
Mechanism: