Module 05
Periodic Properties
Q-1Explain effective nuclear charge.
Answer:
The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. The effective nuclear charge may be approximated by the equation:
Zeff = Z – S
Where Z is the atomic number and S is the number of shielding electrons.
Higher energy electrons can have other lower energy electrons between the electron and the nucleus, effectively lowering the positive charge experienced by the high energy electron.
The shielding effect is the name given to the balance between the attraction between valence electrons and protons and the repulsion between valence and inner electrons. The shielding effect explains the trend in atomic size on the periodic table and also why valence electrons are readily removed from an atom.
Q-2Explain orbital penetration.
Answer:
Penetration describes the proximity to which an electron can approach to the nucleus. In a multi-electron system, electron penetration is defined by an electron's relative electron density near the nucleus of an atom. Electrons in different orbitals have different wave functions and therefore different radial distributions and probabilities. In other words, penetration depends on the shell and subshell. For example, we see that since a 2s electron has more electron density near the nucleus than a 2p electron, it is penetrating the nucleus of the atom more than the 2p electron. The penetration power of an electron, in a multi-electron atom, is dependent on the values of both the shell and subshell.
Within the same shell value, the penetrating power of an electron follows this trend in sub shells:
s>p>d>f
And for different values of shell and subshell, penetrating power of an electron follows this trend:
1s>2s>2p>3s>3p>4s>3d>4p>5s>4d>5p>6s>4f
And the energy of an electron for each shell and subshell goes as follow:
1s<2s<2p<3s<3p<4s<3d<4p
The electron probability density for s-orbitals is highest in the center of the orbital, or at the nucleus. If we imagine a dartboard that represents the circular shape of the s-orbital and if the darts landed in correlation to the probability to where and electron would be found, the greatest dart density would be at the 50 points region but most of the darts would be at the 30 point region. When considering the 1s-orbital, the spherical shell of 53 pm is represented by the 30 point ring.
Electrons which experience greater penetration experience stronger attraction to the nucleus, less shielding, and therefore experience a larger Effective Nuclear Charge, but shield other electrons more effectively.
Q-3Describe electronic configuration.
Answer:
The distribution of electrons of an atom or molecule in atomic or molecular orbitals is called as the electronic configuration. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Electronic configuration describes the specific location of each electrons in atoms or molecules.
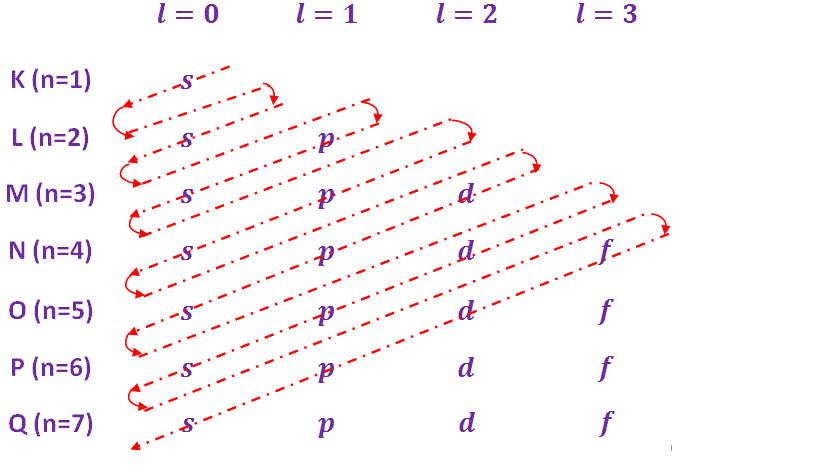
Q-4Explain different orbits and maximum electron occupancy.
Answer:
The maximum number of electrons in any main energy level (shell) is given by, ‘2n2’, where, n is an integer and representing the “principal quantum number”. For different main energy levels the value of ‘n’ and maximum number of electrons are given in table below-
Sl. No. | Energy level or Orbit (shell) | Principal quantum number ‘n’ | Maximum Number of electrons (2n2) |
1 | K | 1 | 2×12 = 2 |
2 | L | 2 | 2×22 = 8 |
3 | M | 3 | 2×32 = 18 |
4 | N | 4 | 2×42 = 32 |
The each main shell (energy level) is subdivided into sub shells. These sub shell are called orbitals. These sub shells /orbitals are designated by s, p, d, f etc. with corresponding orbital quantum number, l = 0, 1, 2, 3, 4,…..(n-1) etc. The number of sub shells in any main shell is equal to “principal quantum number” ‘n’.
Q-5What are atomic radii?
Answer:
Atomic radius is the radius of spherical atoms. Nonbonding atoms have a larger, more undefined radius, so when atomic radius is discussed as a periodic trend, what's usually meant is bonding atomic radius. These are the radius of atoms that are chemically bonded to one another. So, if the bond between two Cl atoms in Cl2 is 1.99 angstroms, we report chlorine's bonding atomic radius as about 0.99 angstroms. Further these values to estimate bond lengths between different elements in molecules.
Q-6What are ionic radii?
Answer:
Ionic radii are the radii of ions of elements. These distances are based on distances between ions in ionic compounds. Cations, or positively charged ions, are smaller than their "parent" atoms. This is because cations are formed when those outermost orbitals are vacated of electrons. This also decreases electron-electron repulsions. Therefore, the resulting ions are smaller as there are not as many occupied orbitals and the effective nuclear charge affecting the remaining electrons increases, pulling electrons in more closely.
Q-7What are ionization energies?
Answer:
Ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic state must absorb to discharge an electron, resulting in a cation.
H(g)→H+(g)+e−(1)(1)H(g)→H+(g)+e−
This energy is usually expressed in kJ/mol.
When considering an initially neutral atom, expelling the first electron will require less energy than expelling the second, the second will require less energy than the third, and so on. Each successive electron requires more energy to be released. This is because after the first electron is lost, the overall charge of the atom becomes positive, and the negative forces of the electron will be attracted to the positive charge of the newly formed ion. The more electrons that are lost, the more positive this ion will be, the harder it is to separate the electrons from the atom.
Q-8Explain the periodic trends of ionization energies.
Answer:
Ionization energies are dependent upon the atomic radius. Since going from right to left on the periodic table, the atomic radius increases, and the ionization energy increases from left to right in the periods and up the groups. Exceptions to this trend are observed for alkaline earth metals (group 2) and nitrogen group elements (group 15). Typically, group 2 elements have ionization energy greater than group 13 elements and group 15 elements have greater ionization energy than group 16 elements. Groups 2 and 15 have completely and half-filled electronic configuration respectively, thus, it requires more energy to remove an electron from completely filled orbitals than incompletely filled orbitals.
Alkali metals (IA group) have small ionization energies, especially when compared to halogens group. In addition to the radius, the number of electrons between the nucleus and the electron in the outermost shell has an effect on the ionization energy as well. This effect, where the full positive charge of the nucleus is not felt by outer electrons due to the negative charges of inner electrons partially canceling out the positive charge, is called shielding. The more electrons shielding the outer electron shell from the nucleus, the less energy required to expel an electron from said atom. The higher the shielding effect the lower the ionization energy. It is because of the shielding effect that the ionization energy decreases from top to bottom within a group. From this trend, Cesium is said to have the lowest ionization energy and Fluorine is said to have the highest ionization energy.
Q-9What are electro negativity? Discuss its periodic trends.
Answer:
It basically indicates the net result of the tendencies of atoms in different elements to attract the bond-forming electron pairs. Electro negativity can be measured on several scales. The most commonly used scale was designed by Linus Pauling. According to this scale, fluorine is the most electronegative element with a value of 4.0 and cesium is the least electronegative element with a value of 0.7.
Periodic Trends of electro negativity: On moving across a period from left to right the nuclear charge increases and the atomic size decreases, therefore the value of electro negativity increases across a period in the modern periodic table.
There is an increase in the atomic number as we move down the group in the modern periodic table. The nuclear charge also increases but the effect of the increase in nuclear charge is overcome by the addition of one shell. Hence, the value of electro negativity decreases as we move down the group. It is a general observation that metals show a lower value of electro negativity as compared to the non-metals.
Q-10Explain the rules for the determination of oxidation state.
Answer:
Rules for the determination of Oxidation States:
- The oxidation state of an uncombined element is zero. This applies regardless of the structure of the element: Xe, Cl2, and large structures of carbon or silicon each have an oxidation state of zero.
- The sum of the oxidation states of all the atoms or ions in a neutral compound is zero.
- The sum of the oxidation states of all the atoms in an ion is equal to the charge on the ion.
- The more electronegative element in a substance is assigned a negative oxidation state. The less electronegative element is assigned a positive oxidation state. Remember that electronegativity is greatest at the top-right of the periodic table and decreases toward the bottom-left.
Q-11Explain the characteristics of hard soft acid base.
Answer:
Characteristics of HSAB:
Hard acid soft acid:
Hard Acid | Soft Acid |
Small iconic radius | Large iconic radius |
High positive charge | Low positive charge |
Low electro negativity | Intermediate electro negativity |
High energy LUMO | Low energy LUMO |
Hard base soft base:
Hard Base | Soft Base |
Small radius | Large radius |
High electro negativity | Intermediate electro negativity |
Weak Polarazibility | High Polarazibility |
High energy HOMO | Low energy HOMO |