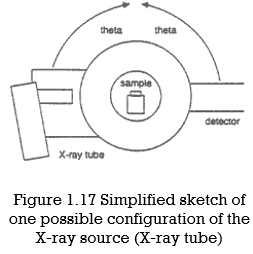
Unit-1
Introduction
Q1) What is geology explain in brief.
A1)
Q2) Explain importance of geology in civil engineering.
A1)
The importance of geology in civil engineering may be briefly outlined as follows:
Q3) Explain scope of geology in civil engineering and geology in construction field.
A3) Scope of geology in civil engineering:
Geology in the construction field
Q3) Give the brief discussion on geological survey of India.
A3) Geological Survey of India (GSI)
Q4) Give the hpc recommendation on engineering geology.
A4)
Following the HPC recommendations, GSI is executing its programs through Mission-Region hybrid matrix mode with its five Mission offices and three Support Systems.
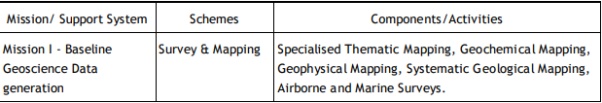
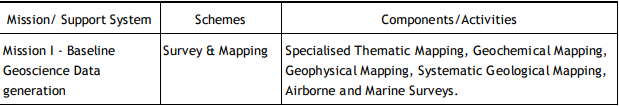
Q5) Explain scope of geological survey of India.
A5) Scope of Geological Survey of India
Q6) What is national institute of rock mechanics.
A6) National Institute of Rock Mechanics (NIRM)
Q7) Explain what is minerals? And what is composition?
A7) Minerals
Composition

Fig. Trigonal (rhombohedral) crystals of quartz.
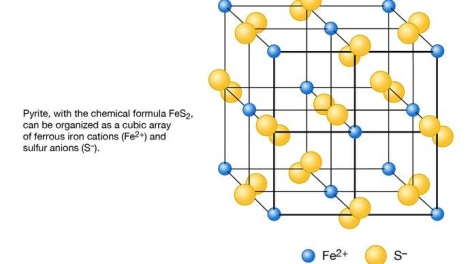
Fig. Schematic representation of the structure of pyrite (FeS2) as based on a cubic array of ferrous iron cations (Fe2+) and sulfur anions (S−).
Q8) Give physical properties of minerals.
A8) Physical properties of minerals
Color, Streak, and Luster
Color

This mineral is shiny, very soft, heavy, and gold in color, and is gold.

Purple quartz, known as amethyst, and clear quartz are the same mineral despite the different colors.
Luster

(a) Diamond has an adamantine luster. (b) Quartz is not sparkly and has a vitreous, or glassy, luster. (c) Sulfur reflects less light than quartz, so it has a resinous luster.
Streak

The streak of hematite across an unglazed porcelain plate is red-brown.
Q9) Explain what is specific gravity, cleavage and fracture.
A9) Specific Gravity
Cleavage and Fracture
Cleavage

Fig. A close-up view of sodium chloride in a water bubble aboard the International Space Station.

Fig. Sheets of mica

Fig. This rough diamond shows its octahedral cleavage.
Q10) Explain what is fracture and crystal shape?
A10) Fracture

Fig. Chrysotile has a splintery fracture.
Crystal Shape
All minerals are crystalline, but only some have the opportunity to exhibit the shapes of their crystals, their crystal forms. Many minerals in an introductory geology lab do not exhibit their crystal form.
If a mineral has space while it grows, it may form natural crystals, with a crystal shape reflecting the geometry of the mineral’s internal crystal lattice. The shape of a crystal follows the symmetry of its crystal lattice. Quartz, for instance, forms six-sided crystals, showing the hexagonal symmetry of its crystal lattice.
There are two complicating factors to remember here:
(1) Minerals do not always form nice crystals when they grow, and
(2) A crystal face is different from a cleavage surface. A crystal face forms during the growth of the mineral. A cleavage surface is formed when the mineral is broken.
Q11) What is susceptibility of minerals to alternation.
A11) Susceptibility of minerals to alteration
Q12) Explain what is optimal mineralogy?
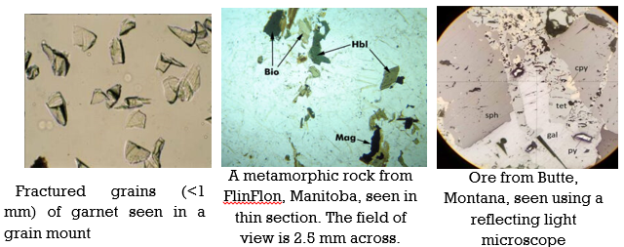
A11) Optical Mineralogy

Fig. Close-up view of microscope lenses and a thin section
Q13) Explain what is scanning electron microscopy?
A13) Scanning Electron Microscopy (SEM)

Fundamental Principles of Scanning Electron Microscopy (SEM)
Scanning Electron Microscopy (SEM) Instrumentation - How Does It Work?
Essential components of all SEMs include the following:
- Power Supply
- Vacuum System
- Cooling system
- Vibration-free floor
- Room free of ambient magnetic and electric fields
SEMs always have at least one detector (usually a secondary electron detector), and most have additional detectors. The specific capabilities of a particular instrument are critically dependent on which detectors it accommodates.
Q14) What is application of scanning electron microscopy?
A14) Applications

The SEM is routinely used to generate high-resolution images of shapes of objects (SEI) and to show spatial variations in chemical compositions:
1) Acquiring elemental maps or spot chemical analyses using EDS,
2) Discrimination of phases based on mean atomic number (commonly related to relative density) using BSE, and
3) Compositional maps based on differences in trace element "activators" (typically transition metal and Rare Earth elements) using CL.
The SEM is also widely used to identify phases based on qualitative chemical analysis and/or crystalline structure. Precise measurement of very small features and objects down to 50 nm in size is also accomplished using the SEM. Backscattered electron images (BSE) can be used for rapid discrimination of phases in multiphase samples. SEMs equipped with diffracted backscattered electron detectors (EBSD) can be used to examine microfabric and crystallographic orientation in many materials.
Strengths and Limitations of Scanning Electron Microscopy (SEM)
Strengths
Limitations
Q15) Explain X-ray diffraction in detail.
A15) X-Ray Diffraction
Theory and Methodology