Unit-2
Petrology-Rock forming processes
Q1) Explain what is petrology, its background and methodology.
A1) Petrology is the branch of geology that studies rocks and the conditions under which they form. Petrology has three subdivisions: igneous, metamorphic, and sedimentary petrology. Igneous and metamorphic petrology are commonly taught together because they both contain heavy use of chemistry, chemical methods, and phase diagrams. Sedimentary petrology is, on the other hand, commonly taught together with stratigraphy because it deals with the processes that form sedimentary rock.
Background
Lithology was once approximately synonymous with petrography, but in current usage, lithology focuses on macroscopic hand-sample or outcrop-scale description of rocks while petrography is the specialty that deals with microscopic details.
In the petroleum industry, lithology, or more specifically mud logging, is the graphic representation of geological formations being drilled through, and drawn on a log called a mud log. As the cuttings are circulated out of the borehole they are sampled, examined (typically under a 10× microscope), and tested chemically when needed.
Methodology
Petrology utilizes the fields of mineralogy, petrography, optical mineralogy, and chemical analysis to describe the composition and texture of rocks. Petrologists also include the principles of geochemistry and geophysics through the study of geochemical trends and cycles and the use of thermodynamic data and experiments to better understand the origins of rocks.
Branches
There are three branches of petrology, corresponding to the three types of rocks: igneous, metamorphic, and sedimentary, and another dealing with experimental techniques:
Igneous petrology focuses on the composition and texture of igneous rocks (rocks such as granite or basalt which have crystallized from molten rock or magma). Igneous rocks include volcanic and plutonic rocks.
Sedimentary petrology focuses on the composition and texture of sedimentary rocks (rocks such as sandstone, shale, or limestone which consist of pieces or particles derived from other rocks or biological or chemical deposits and are usually bound together in a matrix of finer material).
Metamorphic petrology focuses on the composition and texture of metamorphic rocks (rocks such as slate, marble, gneiss, or schist which started as sedimentary or igneous rocks but which have undergone chemical, mineralogical, or textural changes due to extremes of pressure, temperature, or both)
Experimental petrology employs high-pressure, high-temperature apparatus to investigate the geochemistry and phase relations of natural or synthetic materials at elevated pressures and temperatures. Experiments are particularly useful for investigating rocks of the lower crust and upper mantle that rarely survive the journey to the surface in pristine condition. They are also one of the prime sources of information about completely inaccessible rocks such as those in the Earth's lower mantle and the mantles of the other terrestrial planets and the Moon. The work of experimental petrologists has laid a foundation on which modern understanding of igneous and metamorphic processes has been built.
Q2) Explain rock forming process in detail and what is rock cycle?
A2) Rock-forming processes.
The conceptual framework for rock development is provided by a model rock cycle, which is introduced as consisting of primary and secondary loops, corresponding to conventional distinctions between igneous and sedimentary rocks.The formation of primary minerals from magma and the importance of silicates lead naturally into igneous processes and landforms. Alteration and resorption of oceanic crust represent the shortest route through the rock cycle, and there are also strong associations between magma emplacement, subduction, and metamorphism.
The rock cycle
Igneous processes and landforms are associated with subduction zones and hotspots where magma is intruded into existing rocks or extruded and erupted at Earth’s surface. Their distinctive landforms vary according to magma mineralogy and emplacement style. • Intrusive magma solidifies before reaching the surface and is usually therefore dominated by the silicate-rich (felsic), the granitic portion of a melt. It forms an underground network of magma reservoirs, sheets, or columns that may feed surface extrusions or be restricted to thermal diapirs.
Extrusive magma solidifies after reaching the surface as silicate-poor (mafic), basaltic, and often effusive eruptions forming shield volcanoes or basalt plateaux; or as intermediate, andesitic, and often explosive eruptions forming stratovolcanoes.
All rocks may be subjected to alteration due to changes in temperature or pressure, ranging from minor syngenesis or diagenesis to almost complete melting or stigmatization.
Metamorphism lies between these extremes and involves permanent textural or mineralogical alteration without leaving the solid phase. Metamorphism proceeds by textural alteration and/or solid-state recrystallization of constituent minerals under subsurface high temperature and pressure conditions.
Metamorphism is therefore commonly associated with subduction zones, transform plate boundaries, and igneous rock emplacement, and metamorphic rocks comprise up to 70 percent of continental crust.
The infusion of high-temperature fluids and gases associated with magma emplacement or contact metamorphism causes metasomatism or the minero chemical alteration of surrounding rock.
Sediments are the unconsolidated fragments and dissolved debris of other rocks and organisms and are normally transported from their point of origin, during which time their initial characteristics undergo substantial reworking and alteration.
Sediments accumulate by debris deposition and are lithified to form clastic (particulate), chemical (precipitate), and biogenic sedimentary rocks.
Each sediment parcel forms a distinctive facies, which reflects its mode of deposition, and bundles of facies with common genetic origins represent a sediment environment.
Plate tectonics and land surface processes determine the global location of sedimentary basins, which accumulate the detrital products and signature of prevailing environmental conditions.
Q3) Explain oceanic rock cycle in detail and what is specific gravity?
A3) The oceanic rock cycle
Oceans provide unique sites for the formation of new rocks from both magmatic and sedimentary sources. The cold seawater itself weathers sea-bed rocks by hydration or oxidation, forming new mineral species.
Seawater is also recycled through the oceanic crust by hydrothermal circulation associated with mantle convection and mid-ocean ridges, altering the condition of seawater and sea-bed rocks by metasomatism en route.
Ocean basins provide a major sink for the collection, reworking, and recycling of terrigenous sediments, mostly on continental shelf-slope systems.
Long-distance transport of suspended, ice-rafted, and aeolian sediment augments the primarily biogenic muds which accumulate on deep abyssal plains.
Specific gravity
Specific gravity (G) is defined as the ratio between the weight of a substance and the weight of an equal volume of water at 4 °C (39 °F). Thus a mineral with a specific gravity of 2 weighs twice as much as the same volume of water. Since it is a ratio, specific gravity has no units.
The specific gravity of a mineral depends on the atomic weights of all its constituent elements and how the atoms (and ions) are packed together. In mineral series whose species have essentially identical structures, those composed of elements with higher atomic weight have higher specific gravities.
If two minerals (as in the two polymorphs of carbon, namely graphite and diamond) have the same chemical composition, the difference in specific gravity reflects variation in internal packing of the atoms or ions (diamond, with a G of 3.51, has a more densely packed structure than graphite, with a G of 2.23).
Measurement of the specific gravity of a mineral specimen requires the use of a special apparatus. An estimate of the value, however, can be obtained by simply testing how heavy a specimen feels. Most people, from everyday experience, have developed a sense of relative weights for even such objects as nonmetallic and metallic minerals.
For example, borax (G = 1.7) seems light for a nonmetallic mineral, whereas anglesite (G = 6.4) feels heavy. Average specific gravity reflects what a nonmetallic or metallic mineral of a given size should weigh. The average specific gravity for nonmetallic minerals falls between 2.65 and 2.75, which is seen in the range of values for quartz (G = 2.65), feldspar (G = 2.60 to 2.75), and calcite (G = 2.72). For metallic minerals, graphite (G = 2.23) feels light, while silver (G = 10.5) seems heavy.
The average specific gravity for metallic minerals is approximately 5.0, the value for pyrite. With practice using specimens of known specific gravity, a person can develop the ability to distinguish between minerals that have comparatively small differences in specific gravity by merely lifting them.
Although an approximate assessment of specific gravity can be obtained by the hefting of a hand specimen of a specific monomineral, an accurate measurement can only be achieved by using a specific gravity balance.
An example of such an instrument is the Jolly balance, which provides numerical values for a small mineral specimen (or fragment) in the air as well as in water. Such accurate measurements are highly diagnostic and can greatly aid in the identification of an unknown mineral sample.
Q4) Explain Ternary diagram with name.
A4) Ternary diagram
A ternary plot, ternary graph, triangle plot, simplex plot, Gibbs triangle, or de Finetti diagram is a barycentric plot on three variables that sum to a constant. It graphically depicts the ratios of the three variables as positions in an equilateral triangle. It is used in physical chemistry, petrology, mineralogy, metallurgy, and other physical sciences to show the compositions of systems composed of three species.
In population genetics, it is often called a de Finetti diagram. In game theory, it is often called a simplex plot. Ternary plots are tools for analyzing compositional data in the three-dimensional case.
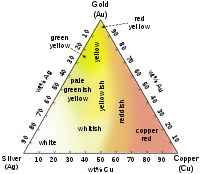
Approximate colors of Ag–Au–Cu alloys in jewelry making
In a ternary plot, the values of the three variables a, b, and c must sum to some constant, K. Usually, this constant is represented as 1.0 or 100%. Because a + b + c = K for all substances being graphed, any one variable is not independent of the others, so only two variables must be known to find a sample's point on the graph: for instance, c must be equal to K − a − b. Because the three numerical values cannot vary independently—there are only two degrees of freedom—it is possible to graph the combinations of all three variables in only two dimensions.
Reading values on the ternary plot
The advantage of using a ternary plot for depicting chemical compositions is that three variables can be conveniently plotted in a two-dimensional graph. Ternary plots can also be used to create phase diagrams by outlining the composition regions on the plot where different phases exist.
Every point on a ternary plot represents a different composition of the three components.
A parallel to a side of the triangle is the locus of points representing systems with constant chemical composition in the component situated in the vertex as opposed to the side.
There are three common methods used to determine the ratios of the three species in the composition.
The first method is an estimation based upon the phase diagram grid. The concentration of each species is 100% (pure phase) in each corner of the triangle and 0% at the line opposite it. The percentage of a specific species decreases linearly with increasing distance from this corner, as seen in figures 3–8.
By drawing parallel lines at regular intervals between the zero line and the corner (as seen in the images), fine divisions can be established for easy estimation of the content of a species. For a given point, the fraction of each of the three materials in the composition can be determined by the first.
For phase diagrams that do not possess grid lines, the easiest way to determine the composition is to set the altitude of the triangle to 100% and determine the shortest distances from the point of interest to each of the three sides. By Viviani's theorem, the distances (the ratios of the distances to the total height of 100%) give the content of each of the species, as shown in figure.
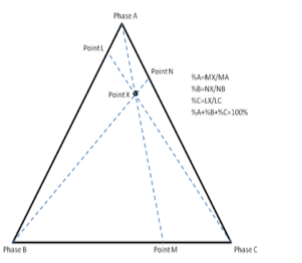
The third method is based upon a larger number of measurements but does not require the drawing of perpendicular lines. Straight lines are drawn from each corner, through the point of interest, to the opposite side of the triangle. The lengths of these lines, as well as the lengths of the segments between the point and the corresponding sides, are measured individually. Ratios can then be determined by dividing these segments by the entire corresponding line as shown in figure 2.3 (The sum of the ratios should add to 1).
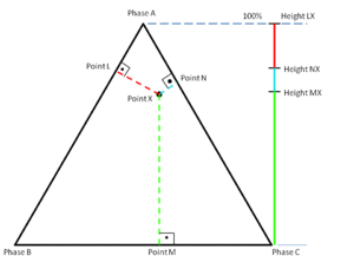
Altitude method
Intersection method
An example ternary diagram, without any points, plotted.
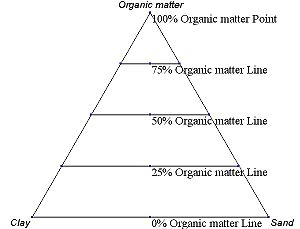
An example ternary diagram, showing increments along the first axis.
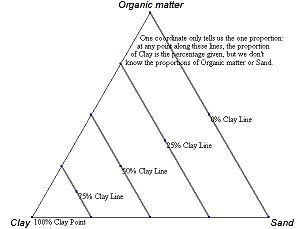
An example ternary diagram, showing increments along the second axis.
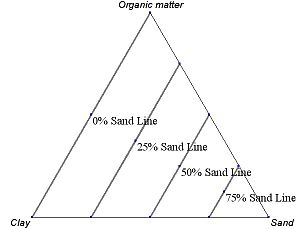
An example ternary diagram, showing increments along the third axis.
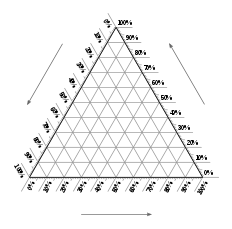
Figure: Empty ternary plot
Q5) Explain in detail igneous rocks.
A5) Igneous Rocks
Igneous rock is formed when liquid rock freezes into solid rock. This molten material is called magma when it is in the ground and lava when it is on the surface. Only the Earth’s outer core is liquid; the Earth’s mantle and crust are naturally solid. However, there are a few minor pockets of magma that form near the surface where geologic processes cause melting.
It is this magma that becomes the source for volcanoes and igneous rocks. This chapter will describe the classification of igneous rocks, the unique processes that form magmas, types of volcanoes and volcanic processes, volcanic hazards, and igneous landforms.

Figure: The lava flow in Hawaii
Lava cools quickly on the surface of the earth and forms tiny microscopic crystals. These are known as fine-grained extrusive, or volcanic, igneous rocks. Extrusive rocks are often vesicular, filled with holes from escaping gas bubbles.
Volcanism is the process in which lava erupts. Depending on the properties of the lava that is erupted, the volcanism can be drastically different, from smooth and gentle to dangerous and explosive. This leads to different types of volcanoes and different volcanic hazards.
In contrast, magma that cools slowly below the earth’s surface forms larger crystals which can be seen with the naked eye. These are known as coarse-grained intrusive, or plutonic, igneous rocks. This relationship between cooling rates and grain sizes of the solidified minerals in igneous rocks is important for interpreting the rock’s geologic history.
Materials Produced by Volcanic Eruptions
Volcanic eruptions produce three types of materials: gas, lava, and fragmented debris called tephra.
Volcanic Gas
Magma contains gas. At high pressures, the gases are dissolved within magma. However, if the pressure decreases, the gas comes out of the solution, forming bubbles. This process is analogous to what happens when a pop bottle is opened. Pop is bottled under pressure, forcing carbon dioxide gas to dissolve into the fluid. As a result, a bottle of pop that you find on the supermarket shelf will have few to no bubbles. If you open the bottle, you decrease the pressure within it. The pop will begin to fizz as carbon dioxide gas comes out of the solution and forms bubbles.
The main component of volcanic gas emissions is water vapor, followed by carbon dioxide (CO2), sulfur dioxide (SO2), and hydrogen sulfide (H2S).
Volcanoes release gases when erupt, and through openings called fumaroles (Figure). They can also release gas into soil and groundwater.

Figure: A fumarole at PuʻuʻŌʻō Crater, Hawaii.
Q6) Explain what is lava.
A6) Lava
The ease with which lava flows and the structures it forms depends on how much silica and gas the lava contains. The more silica, the more polymerization (formation of long molecules) occurs, stiffening the lava. The stiffness of lava is described in terms of viscosity– lava that flows easily has low viscosity, and lava that is sticky and stiff has high viscosity.
In general, high-silica lava contains more gas than low-silica lava. When the gas forms into bubbles, viscosity increases further. Consider the pop analogy again. If you were to shake the bottle vigorously and then open it, the pop would come gushing out in a thick, frothy flow. In contrast, if you took care to not shake the bottle before opening it, you could pour out a thin stream of fluid.
Chemical Composition Affects the Thickness and Shape of Lava Flows
The thickness and shape of a lava flow depend on its viscosity. The greater the viscosity, the thicker the flow, and the shorter the distance it travels before solidifying. Highly viscous lava might not flow very far at all and simply accumulate as a bulge, called a lava dome, in a volcano’s crater. Figure 2.12 shows a dome formed from rhyolitic lava in the crater of Mt. St. Helens.

Figure: Lava dome in the crater of Mt. St. Helens.
Less viscous rhyolitic lava can travel further, as with the thick flow in Figure (right). The left of Figure shows thin streams of freely-flowing, low-silica, low-viscosity basaltic lava.
 Figure: Lava flows.
Figure: Lava flows.
Low-viscosity basaltic lava flows may travel extended distances if they move through conduits called lava tubes. These are tunnels within older solidified lava flows. Figure(top) shows a view into a lava tube through a hole in the overlying rock, called a skylight. Figure(bottom) shows the interior of a lava tube, with a person for scale. Lava tubes form naturally and readily because flowing mafic lava preferentially cools near its margins, forming solid lava levées that eventually close over the top of the flow. Lava within tubes can flow for 10s of km because the tubes insulate the lava from the atmosphere and slow the rate at which the lava cools. The Hawai’ian volcanoes are riddled with thousands of old, drained lava tubes, some as long as 50 km.
 Figure: Lava tubes.
Figure: Lava tubes.
Q7) Explain pyroclastic materials, volcanic ash and lapilli.
A7) Pyroclastic Materials
The pop bottle analogy illustrates another key point about gas bubbles in the fluid, which is that the bubbles can propel fluid. In the same way that shaking a pop bottle to make more bubbles will cause pop to gush out when the bottle is opened, gas bubbles can violently propel lava and other materials from a volcano, creating an explosive eruption.
Collectively, loose material thrown from a volcano is referred to as tephra. Individual fragments are referred to in general terms as pyroclasts, so sometimes tephra is also referred to as pyroclastic debris. Pyroclasts are classified according to size.
Volcanic Ash
Particles less than 2 mm in diameter are called volcanic ash. Volcanic ash consists of small mineral grains and glass. Figure 2.15 shows volcanic ash on three scales: in the upper left is ash from the 2010 eruption of Eyjafjallajökull in Iceland. The image was taken with a scanning electron microscope at approximately 1000 times magnification.
In the upper right is ash from the 1980 eruption of Mt. St. Helens, collected in Yakima, Washington, about 137 km northeast of Mt. St. Helens. Individual particles are under 1 mm in size. Figure 2.15 (bottom) shows a village near Mt. Merapi in Indonesia dusted in ash after an eruption in 2010.
 Figure: Volcanic ash.
Figure: Volcanic ash.
Lapilli
Fragments with dimensions between 2 mm and 64 mm are classified as lapilli. Figure 2.16 (upper left) shows lapilli at the ancient city of Pompeii, which was buried when Mt. Vesuvius erupted in 79 C.E. Figure 2.16 (lower left) is a form of lapilli called Pele’s tears, named after the Hawai’ian deity Pele. Pele’s tears form when droplets of lava cool quickly as they are flung through the air. Rapidly moving through the air may draw the Pele’s tears out into long threads called Pele’s hair (Figure 2.16, right). The dark masses in Figure 2.16 (right) within Pele’s hair are Pele’s tears.
 Figure: Lapilli are pyroclasts ranging between 2 mm and 64 mm in size
Figure: Lapilli are pyroclasts ranging between 2 mm and 64 mm in size
Blocks and Bombs
Fragments larger than 64 mm are classified as blocks or bombs, depending on their origin. Blocks are solid fragments of the volcano that form when an explosive eruption shatters the pre-existing rocks.
Bombs form when lava is thrown from the volcano and cools as it travels through the air. Traveling through the air may cause the lava to take on a streamlined shape, as with the example in Figure 2.17
 Figure: Volcanic bomb with a streamlined shape.
Figure: Volcanic bomb with a streamlined shape.
Q8) Explain types of volcanic eruption in detail.
A8) Types of Volcanic Eruptions
There are four types of eruptions with properties determined mostly by the silica content of the magma, and the amount of gas it contains. In order of increasing explosiveness, these are Hawai’ian, Strombolian, Vulcanian, and Plinian eruptions. Any composition of magma can have an explosive eruption if the magma suddenly encounters water.
Hot magma contacting groundwater or seawater causes the water to flash to steam. Explosive eruptions driven by water are called hydrovolcanic (or phreatic) eruptions.
Hawai‘ian Eruptions
Hawai‘ian eruptions are named after the characteristic eruptions of volcanoes of the Hawai‘ian islands. Hawai‘ian eruptions are effusive (flowing) rather than explosive because they erupt low-viscosity basaltic lava.
Hawai‘ian eruptions form shield volcanoes and can also take the form of fissure eruptions. Fissure eruptions occur when lava erupts from long cracks in the ground rather than from a central vent.
Figure shows examples from two eruptions on of Hawai‘i. In the upper left and right are images from the November 1959 eruption of KīlaueaIki Crater. The upper left shows a fissure eruption and effusive flow of lava. Burning trees appear as bright spots toward the bottom of the photo. Figure 2.18 (right) shows a lava fountain reaching 425 m above KīlaueaIki Crater. U. S. Geological Survey scientists reported that volcanic bombs up to 60 cm across smashed the guard rail and dented the asphalt on the road. Figure 2.18 (lower left) shows Hawaiian Volcano Observatory (HVO) scientists making a quick getaway, with lava fountains from Mauna Loa Volcano in the background.

Figure: Hawai‘ian eruptions.
The photographs of the KīlaueaIki Crater and Mauna Loa Volcano eruptions make the point that while Hawai‘ian eruptions are considered “gentle” eruptions, this is a relative term. “Gentle” eruptions range from lava flows that can be safely sampled by trained personnelto lava fountains that soar hundreds of meters above the treetops and rain large and dangerous rocks upon the surroundings.
Strombolian Eruptions
Strombolian eruptions, named for Mt. Stromboli in Italy, occur when basaltic lava has a higher viscosity and higher gas content. The sticky lava is ejected in loud, violent, but short-lived splattery eruptions. Clumps of gas-rich lava thrown 10s to 100s of meters in the air accumulate as scoria in a pile around the vent, forming cinder cones. Figure 2.19 shows a strombolian eruption in the crater of Mt. Etna. A smaller cinder cone is forming around the vent as lava sputters out of it.
 Figure: Strombolian eruption of Mt. Etna.
Figure: Strombolian eruption of Mt. Etna.
Vulcanian Eruptions
Vulcanian eruptions get their name from the volcanic Italian island of Vulcano, which itself takes the name of the Roman god of fire, Vulcan. In Roman mythology, Vulcan was the maker of armor and weaponry for the gods, and volcanic eruptions were attributed to him working in his forge.
Vulcanian eruptions are far more explosive than Strombolian eruptions and can blast tephra and gas to a height of 5 to 10 km. The explosiveness is related to a build-up of pressure as the higher viscosity of intermediate silica content lava restricts the escape of gas. Vulcanian eruptions produce large quantities of ash in addition to blocks and bombs.
The Vulcanian eruption of Mt. Pelée on the island of Martinique in 1902 resulted in the first detailed documentation by geologists of a devastating phenomenon that is now referred to as a pyroclastic flow (Fig. 2.20). Volcanic debris from the collapse of a lava dome on Mt. Pelée combined with hot gas to form a searing avalanche that raced down the mountain, over the city of St. Pierre, and into the harbor.
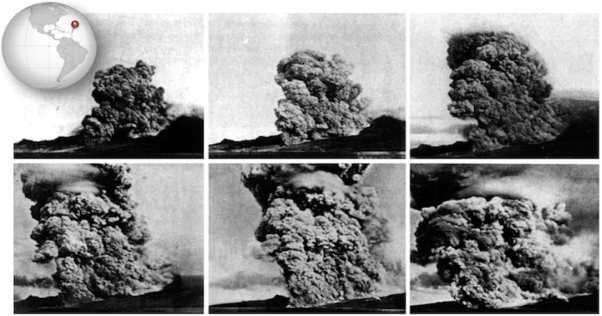 Figure: A series of photos taken by Alfred Lacroix during the eruption of Mt. Pelée
Figure: A series of photos taken by Alfred Lacroix during the eruption of Mt. Pelée
The French geologist Alfred Lacroix described what he saw as a “nuée ardente,” or thick fiery cloud.
In some cases, pyroclastic flows travel at speeds greater than 700 km/h. They can travel rapidly because they behave like fluid, and can also ride on a cushion of hot gas. The ruins of St. Pierre look as though the top of the city were shaved off, and that is effectively what happened as the pyroclastic flow rushed across it, buoyed by gas.
The vast majority of fatalities from the eruption were caused by the heat of pyroclastic flow. Examination of the ruins of St. Pierre revealed that glass had melted, but copper had not, putting the temperature at between 700 ºC and 1000 ºC (1292 ºF to 1832 ºF).
Q9) What is plinian eruption, hydroholcanic eruption and hot spring
A9) Plinian Eruptions
Plinian eruptions are explosive eruptions of intermediate to felsic lava and can form eruptive columns up to 45 km high. The origin of the name is the eruption of Vesuvius in 79 CE, which buried the towns of Pompeii and Herculaneum.
The Roman admiral Gaius Plinius Secundus, also known as Pliny the Elder, attempted a rescue mission when he saw the column of ash and debris above Vesuvius, but died of unknown causes without being able to reach Herculaneum.
A more recent Plinian eruption was that of Mt. Redoubt on April 21, 1990, shown in Figure. Pyroclastic flows resulted, as did lahars, landslides that formed when glaciers melted and turned volcanic ash into the mud. The shape of the eruptive column, with parts of the column appearing to spread out in flat layers at different levels, reflects differences in atmospheric characteristics.
 Figure: Plinian eruption of Mt. Redoubt in Alaska on April 21, 1990
Figure: Plinian eruption of Mt. Redoubt in Alaska on April 21, 1990
Hydrovolcanic (Phreatic) Eruptions
Hydrovolcanic eruptions can be far more explosive than Plinian eruptions. They occur when water in the form of groundwater, seawater, or even melting glacial ice or snow comes into contact with magma. The heat from the magma changes water suddenly to steam, which can expand to more than a thousand times the original volume of water. The sudden expansion results in an explosive force that can blast a volcano to pieces and create large amounts of volcanic ash.
In April of 2010, activity by the Icelandic volcano Eyjafjallajökull (Figure 2.22) melted the glacier above it, releasing large quantities of water and triggering a hydrovolcanic eruption. Ash rose in a plume 10 km high and was blown westward and into the skies over Europe. Volcanic ash can damage or destroy aircraft engines, so precaution was taken to prohibit air travel for 5 days. The enormous economic impact of stopping flights has led to numerous studies about the best way to deal with similar events with volcanic ash in the future.
 Figure: Hydrovolcanic eruption of Eyjafjallajökull in April of 2010.
Figure: Hydrovolcanic eruption of Eyjafjallajökull in April of 2010.
Hot Springs and Geysers
Hot springs and geysers are manifestations of volcanic activity. They result from the interaction of groundwater with magma or with solidified but still-hot igneous rocks at shallow depths.
Hot Springs
When hot water gently rises to the surface, it creates a hot spring. A hot spring forms where a crack in the Earth allows water to reach the surface after being heated underground. Many hot springs are used by people and animals as natural hot tubs. Some people believe that hot springs can cure illnesses. Hot springs are found all over the world, even in Antarctica.
Geysers
Geysers are also created by water that is heated beneath the Earth’s surface. The water may become superheated by magma. It becomes trapped in a narrow passageway. The heat and pressure build as more water is added. When the pressure is too much, the superheated water bursts out onto the surface. This is a geyser.
Yellowstone National Park in the United States is one of the most famous areas of hot springs and geysers in the world. The total heat flux from these thermal features is estimated to be 300 megawatts (300 million watts).
The last great eruption at Yellowstone occurred about 630,000 years ago when some 1,000 cubic km (240 cubic miles) of rhyolitic pumice and ash were ejected in huge pyroclastic flows and resulted in the formation of a caldera—a large circular or oval depression caused by the collapse of the surface following magma removal—approximately 45 by 75 km (28 by 47 miles) in size.
Yellowstone Lake now occupies part of this giant caldera. Since that last great outburst, about 1,200 cubic km (288 cubic miles) of rhyolite lava flows and domes have erupted in numerous smaller events. The cooling roots of such past eruptions, or possibly the new intrusions of magma at shallow depth, are the heat sources for the Yellowstone hot springs and geysers.
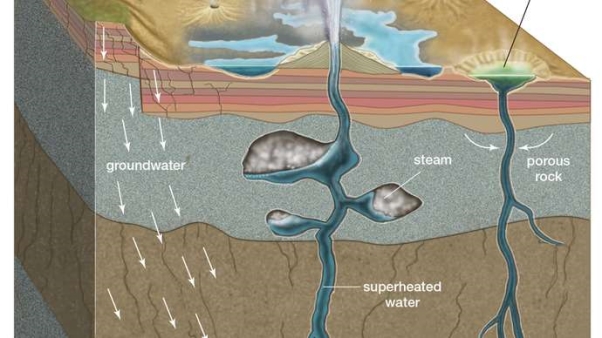
Groundwater percolates through the porous rock into fractures deep underground, where heat from a nearby magma chamber superheats the pressurized water to a temperature above the boiling point of water at surface pressure. In hot springs the rising superheated water is cooled below the boiling point by groundwater before reaching the surface.
In geysers, the superheated water collects in underground pockets. There a small drop in pressure caused by the release of water at the surface flashes the superheated water into steam, which expands and ejects a column of steam and water into the air. When the supply of steam and hot water is exhausted, the spouting stops and the cycle begins again.
Q10) What are the characteristics of magma?
A10) Characteristics of Magma
Types of Magma
Types of magma are determined by the chemical composition of the magma. Three general types are recognized:
Basaltic magma -- SiO2 45-55 wt%, high in Fe, Mg, Ca, low in K, Na
Andesitic magma -- SiO2 55-65 wt%, intermediate. in Fe, Mg, Ca, Na, K
Rhyolitic magma -- SiO2 65-75%, low in Fe, Mg, Ca, high in K, Na
Gases in Magmas
At depth in the Earth, nearly all magmas contain gas dissolved in the liquid, but the gas forms a separate vapor phase when pressure is decreased as magma rises toward the surface. This is similar to carbonated beverages which are bottled at high pressure. The high pressure keeps the gas in the solution in the liquid, but when pressure is decreased, like when you open the can or bottle, the gas comes out of the solution and forms a separate gas phase that you see as bubbles. Gas gives magmas their explosive character because the volume of gas expands as pressure is reduced. The composition of the gases in magma are:
Mostly H2O (water vapor) with some CO2 (carbon dioxide)
Minor amounts of Sulfur, Chlorine, and Fluorine gases
The amount of gas in magma is also related to the chemical composition of the magma. Rhyolitic magmas usually have higher dissolved gas contents than basaltic magmas.
Temperature of Magmas
The temperature of magmas is difficult to measure (due to the danger involved), but laboratory measurement and limited field observation indicate that the eruption temperature of various magmas is as follows:
Basaltic magma - 1000 to 1200oC
Andesitic magma - 800 to 1000oC
Rhyolitic magma - 650 to 800oC.
Viscosity of Magmas
Viscosity is the resistance to flow (opposite of fluidity). Viscosity depends primarily on the composition of the magma, and temperature.
Higher SiO2 (silica) content magmas have a higher viscosity than lower SiO2 content magmas (viscosity increases with increasing SiO2 concentration in the magma).
Lower temperature magmas have a higher viscosity than higher temperature magmas (viscosity decreases with increasing temperature of the magma).
Thus, basaltic magmas tend to be fairly fluid (low viscosity), but their viscosity is still 10,000 to 100,0000 times more viscous than water. Rhyolitic magmas tend to have an even higher viscosity, ranging between 1 million and 100 million times more viscous than water. (Note that solids, even though they appear solid have a viscosity, but it is very high, measured as the trillions of time the viscosity of water). Viscosity is an important property in determining the eruptive behavior of magmas.
Summary Table | |||||
Magma Type | Solidified Rock | Chemical Composition | Temperature | Viscosity | Gas Content |
Basaltic | Basalt | 45-55 SiO2 %, high in Fe, Mg, Ca, low in K, Na | 1000 – 1200 oC | 10 – 103 PaS | Low |
Andesitic | Andesite | 55-65 SiO2 %, intermediate in Fe, Mg, Ca, Na, K | 800 – 1000 oC | 103 – 105 PaS | Intermediate |
Rhyolitic | Rhyolite | 65-75 SiO2 %, low in Fe, Mg, Ca, high in K, Na. | 650 – 800 oC | 105 – 109 PaS | High |
Q11) Explain the division of rock in detail.
A11) Division of rocks
Based on the depth of formation
Igneous rocks are those that solidify from magma, a molten mixture of rock-forming minerals and usually volatiles such as gases and steam. Since their constituent minerals are crystallized from molten material, igneous rocks are formed at high temperatures. They originate from processes deep within the Earth—typically at depths of about 50 to 200 kilometers (30 to 120 miles)—in the mid-to lower-crust or the upper mantle. Igneous rocks are subdivided into two categories: intrusive (emplaced in the crust), and extrusive (extruded onto the surface of the land or ocean bottom), in which case the cooling molten material is called lava.
Sedimentary rocks are those that are deposited and lithified (compacted and cemented together) at the Earth’s surface, with the assistance of running water, wind, ice, or living organisms. Most are deposited from the land surface to the bottoms of lakes, rivers, and oceans. Sedimentary rocks are generally stratified—i.e., they have layering. Layers may be distinguished by differences in color, particle size, type of cement, or internal arrangement.
Metamorphic rocks are those formed by changes in preexisting rocks under the influence of high temperature, pressure, and chemically active solutions. The changes can be chemical (compositional) and physical (textural) in character. Metamorphic rocks are often formed by processes deep within the Earth that produce new minerals, textures, and crystal structures. The recrystallization that takes place does so essentially in the solid-state, rather than by complete remelting, and can be aided by ductile deformation and the presence of interstitial fluids such as water. Metamorphism often produces apparent layering, or banding, because of the segregation of minerals into separate bands. Metamorphic processes can also occur at the Earth’s surface due to meteorite impact events and pyro-metamorphism taking place near burning coal seams ignited by lightning strikes.
Types of igneous rocks can be differentiated according to their texture, color, and composition. The difference in these three parameters depends on the environment of deposition and the chemistry of magmas. Below is an explanation of each parameter as mentioned above.
Based on Chemical composition
Composition refers to a rock’s chemical and mineral make-up. For igneous rock, the composition is divided into four groups: felsic, intermediate, mafic, and ultramafic. These groups refer to differing amounts of silica, iron, and magnesium found in the minerals that make up the rocks.
It is important to realize these groups do not have sharp boundaries in nature, but rather lie on a continuous spectrum with many transitional compositions and names that refer to specific quantities of minerals. As an example, granite is a commonly-used term but has a very specific definition that includes exact quantities of minerals like feldspar and quartz. Rocks labeled as ‘granite’ in laymen applications can be several other rocks, including syenite, tonalite, and monzonite.
Texture
When magma cools slowly large crystals form and rock forms phaneritic texture on the other hand if magma cools fast then small crystals form sometimes a glassy texture where no minerals form can be achieved this way. It is based on the textural difference that igneous rocks can be divided into either extrusive or intrusive rocks. Examples of both extrusive and extrusive rocks are given in Figure 2.24. Intrusive are rocks that form by magma solidifying before reaching the surface hence forming coarse-grained texture while extrusive are those that magma solidifies on the surface forming fine-grained rocks.
Colour
A rock with majorly dark minerals form mafic rocks but with more fractionation, during magma cooling, lighter-colored minerals can form based on the Bowens series. Based on this color difference the rocks can be either mafic or felsic in Figure 2.24 shows that as you move from right to left you have more ultra-mafic due to fractionation.
Composition
Igneous rocks can also be classified based on chemistry. This is mainly based on silica content as highlighted in Figure 2.24. When silica is above 75% main minerals that form are feldspars while with reduction of silica more mafic minerals form, hence the basis for rock difference.

Figure: Types of igneous rocks based on texture, color, and chemistry
Q12) Explain the texture of rock in detail.
A12) Texture
Phaneritic Texture
Phaneritic textured rocks are comprised of large crystals that are visible with or without a hand lens or binocular microscope. The entire rock is made up of large crystals, which are generally 1/2 mm to several centimeters in size; no fine matrix material is present. This texture forms by the slow cooling of magma deep underground in the plutonic environment.
Aphanitic Texture
Aphanitic texture consists of small crystals that cannot be seen by the eye with or hand lens. The entire rock is made up of small crystals, which are generally less than 1/2 mm in size. This texture results from rapid cooling in volcanic or hypabyssal (shallow subsurface) environments.
Porphyritic Texture
Porphyritic rocks are composed of at least two minerals having a conspicuous (large) difference in grain size. The larger grains are termed phenocrysts and the finer grains either matrix or groundmass (see the drawing below and image to the left). Porphyritic rocks are thought to have undergone two stages of cooling; one at the depth where the larger phenocrysts formed and a second at or near the surface where the matrix grains crystallized.
Glassy Texture
Glass-textured igneous rocks are non-crystalline meaning the rock contains no mineral grains. Glass results from cooling that is so fast that minerals do not have a chance to crystallize. This may happen when magma or lava comes into quick contact with much cooler materials near the Earth's surface. Pure volcanic glass is known as obsidian.
Vesicular Texture
This term refers to vesicles (cavities) within the igneous rock. Vesicles are the result of gas expansion (bubbles), which often occurs during volcanic eruptions. Pumice and scoria are common types of vesicular rocks.
Fragmental (Pyroclastic) Texture
Pyroclastic is rocks blown out into the atmosphere during violent volcanic eruptions. These rocks are collectively termed fragmental. If you examine a fragmental volcanic rock closely you can see why. You will note that it is comprised of numerous grains or fragments that have been welded together by the heat of volcanic eruption. If you run your fingers over the rock it will often feel grainy like sandpaper or sedimentary rock. You might also spot shards of glass embedded in the rock.
Classification of metamorphic rocks depends on textures and their degree of metamorphism. Three kinds of criteria are normally employed in the classification of metamorphic rock. These are:
Mineralogical
The most abundant minerals are used as a prefix to a textural term. Thus, a schist containing biotite, garnet, quartz, and feldspar, would be called biotite-garnet schist. A gneiss containing hornblende, pyroxene, quartz, and feldspar would be called a hornblende-pyroxene gneiss. Schist containing porphyroblasts of K-feldspar would be called K-spar porphyroblastic schist.
Chemical
If the general chemical composition can be determined from the mineral assemblage, then a chemical name can be employed. For example, a schist with a lot of quartz and feldspar and some garnet and muscovite would be called garnet-muscovite quartzo-feldspathic schist. Schist consisting mostly of talc would be called talc-magnesian schist.
Texture
Most metamorphic textures involve foliation. Foliation is generally caused by a preferred orientation of sheet silicates. If a rock has a slatey cleavage as its foliation, it is termed a slate, if it has a phyletic foliation, it is termed a phyllite, if it has a shistose foliation, and it is termed schist. A rock that shows a banded texture without a distinct foliation is termed a gneiss. All of these could be porphyroblastic (i.e. could contain porphyroblasts). A rock that shows no foliation is called a hornfels if the grain size is small, and a granulite if the grain size is large and individual minerals can be easily distinguished with a hand lens.
Q13) Explain the field classification of rock in detail.
A13) Field Classification Chart

Classification of Igneous Rocks
Igneous rocks can be divided into four categories based on their chemical composition: felsic, intermediate, mafic, and ultramafic. The diagram of Bowen’s reaction series shows that differences in chemical composition correspond to differences in the types of minerals within an igneous rock. Igneous rocks are given names based on the proportion of different minerals they contain. Figure 2.26 is a diagram with the minerals from Bowen’s reaction series and is used to decide which name to give an igneous rock.
 Figure: Classification diagram for igneous rocks.
Figure: Classification diagram for igneous rocks.
To see how Figure works, first notice the scale in percent along the vertical axis. The interval between each tick mark represents 10% of the minerals within rock by volume. Igneous rock can be represented as a vertical line drawn through the diagram, and the vertical scale used to break down the proportion of each mineral it contains. For example, the arrows in the mafic field of the diagram represent a rock containing 48% pyroxene and 52% plagioclase feldspar. An igneous rock at the boundary between the mafic and ultramafic fields (marked with a vertical dashed line) would have approximately 20% olivine, 50% pyroxene, and 30% Ca-rich plagioclase feldspar by volume
Felsic refers to a predominance of the light-colored (felsic) minerals feldspar and silica in the form of quartz. These light-colored minerals have more silica as a proportion of their overall chemical formula. Minor amounts of dark-colored (mafic) minerals like amphibole and biotite mica may be present as well. Felsic igneous rocks are rich in silica (in the 65-75% range, meaning the rock would be 65-75% weight percent SiO2) and poor in iron and magnesium.
Intermediate is a composition between felsic and mafic. It usually contains roughly-equal amounts of light and dark minerals, including light grains of plagioclase feldspar and dark minerals like amphibole. It is intermediate in silica in the 55-60% range.
Mafic refers to an abundance of ferromagnesian minerals (with magnesium and iron, chemical symbols Mg and Fe) plus plagioclase feldspar. It is mostly made of dark minerals like pyroxene and olivine, which are rich in iron and magnesium and relatively poor in silica. Mafic rocks are low in silica, in the 45-50% range.
Ultramafic refers to the extremely mafic rocks composed of mostly olivine and some pyroxene which have even more magnesium and iron and even less silica. These rocks are rare on the surface, but makeup peridotite, the rock of the upper mantle. It is poor in silica, in the 40% or less range.
Q14) Explain UIGS classification of rock and granite in detail.
A14) IUGS Classification
In the field, a simple field-based classification must be used. This is usually based on mineralogical content and texture. For plutonic rocks, the IUGS system of classification can be used. For volcanic rocks, the following table can be used.
Simple Field Classification of Volcanic Rocks | ||
Rock Name | Essential Minerals* | Other Minerals (may or may not be present) |
Basalt | Olivine | Cpx, Opx, Plag. |
Basanite | Olivine + Feldspathoid (Nepheline/ Leucite) | Cpx, Plag. |
Andesite | No olivine, abundant Plagioclase | Cpx, Opx, Hornblende |
Trachyte | Sanidine + Plagioclase | Na-Cpx, Hornblende, Biotite |
Dacite | Plagioclase + Hornblende | Cpx, Opx, Biotite |
Rhyolite | Quartz | Sanidine, Biotite, Plag., Hornblende, Cpx, Opx |
* The amount of glass in the groundmass increases, in general, from the top to the bottom of the chart. | ||
Once the rocks are brought back to the laboratory and thin sections can be made, these are examined, mineralogical content can be more precisely determined, and refinements in the mineralogical and textural classification can be made.
Chemical analyses can be obtained, and chemical classification, such as the LeBas et al., IUGS chemical classification of volcanic rocks (based on total alkalies [Na2O + K2O] vs. SiO2 diagram shown in fig.

Note that at each stage of the process, the classification may change, but it is important to keep in mind that each stage has limitations, and that classification at each stage is to describe the rock, not only for the individual investigator but anyone else. Thus, the classification scheme should be employed consistently so that later investigators can understand what you are talking about at each stage of the process.
Granite
Granite is the most widespread of igneous rocks, underlying much of the continental crust. Granite is intrusive igneous rock. Intrusive rocks form from molten material (magma) that flows and solidifies underground, where magma cools slowly. Eventually, the overlying rocks are removed, exposing the granite. Granites usually have a coarse texture (individual minerals are visible without magnification), because the magma cools slowly underground, allowing larger crystal growth.
Granites are most easily characterized as light-colored and coarse-grained as a result of cooling slowly below the surface. Color variation is a response to the percent of each mineral found in the sample. The crystals in granite provide a variety of mixed colors — feldspar (pink or red), mica (dark brown or black), quartz (clear pink, white, or black), and amphibole (black).
Granite is high in quartz (about 25%), feldspar, and mica. It is widely used for architectural facades, construction materials, ornamental stone, and monuments. Over 40% of dimension stone quarried is granite. Crushed granite is used as a durable construction material in asphalt and concrete used in highway and infrastructure projects.


Description
Granite is the most widespread of igneous rocks, underlying much of the continental crust. Granite is intrusive igneous rock. Intrusive rocks form from molten material (magma) that flows and solidifies underground, where magma cools slowly. Eventually, the overlying rocks are removed, exposing the granite. Granites usually have a coarse texture (individual minerals are visible without magnification), because the magma cools slowly underground, allowing larger crystal growth.
Granites are most easily characterized as light-colored and coarse-grained as a result of cooling slowly below the surface. Color variation is a response to the percent of each mineral found in the sample. The crystals in granite provide a variety of mixed colors — feldspar (pink or red), mica (dark brown or black), quartz (clear pink, white, or black), and amphibole (black).
Granite is high in quartz (about 25%), feldspar, and mica. It is widely used for architectural facades, construction materials, ornamental stone, and monuments. Over 40% of dimension stone quarried is granite. Crushed granite is used as a durable construction material in asphalt and concrete used in highway and infrastructure projects.
Relation to Mining
Granite is mined as either crushed stone or dimension stone mainly using open-pit mining methods. Crushed granite represents 16% of the total crushed stone produced in the U.S., and it is the second-most utilized crushed stone in the U.S. Crushed limestone is by far the most commonly used crushed rock in the U.S., representing 70% of total crushed rock consumption. Crushed granite is used in road construction and railroad beds. Larger pieces of granite are used to stabilize the land around roadways to minimize and even eliminate soil erosion.
Uses
There is an enormous abundance of granite throughout the United States, so it is not a surprise that a significant amount of granite is used in crushed stone applications. Crushed granite represents 16% of the total crushed stone produced in the U.S., and it is the second-most utilized crushed stone in the U.S. Crushed limestone is by far the most commonly used crushed rock in the U.S., representing 70% of total crushed rock consumption. The 16% represented by crushed granite (265,000 tons per year) is used in road construction and railroad beds. Larger pieces of granite are used to stabilize the land around roadways to minimize and even eliminate soil erosion.
Granite is used extensively as dimension stone. It is used in the construction of buildings, both as building blocks and as veneers on frame structures. Because it can be smoothed to a very high polish, granite has found extensive use in memorials, headstones, monuments, carved decorations on buildings, statues, and the like. Approximately 1.5 million tons of dimension stone are produced annually in the United States. Of this, granite accounts for over 400,000 tons (27%), second only to limestone.
Q15) What do you mean by Rhyolite, explain in detail.
A15) Rhyolite
Rhyolite is an extrusive igneous rock with very high silica content. It is usually pink or gray with grains so small that they are difficult to observe without a hand lens. Rhyolite is made up of quartz, plagioclase, and sanidine, with minor amounts of hornblende and biotite. Trapped gases often produce vugs in the rock. These often contain crystals, opal, or glassy material.
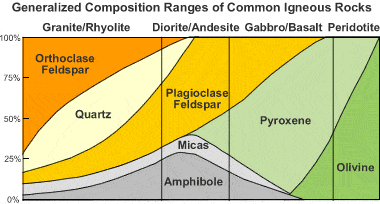
Figure: Igneous rock composition chart: This chart shows that rhyolite is typically composed of orthoclase, quartz, plagioclase, micas, and amphiboles.
Many rhyolites form from granitic magma that has partially cooled in the subsurface. When these magmas erupt, a rock with two-grain sizes can form. The large crystals that formed beneath the surface are called phenocrysts, and the small crystals formed at the surface are called groundmass.
Rhyolite usually forms in continental or continent-margin volcanic eruptions where granitic magma reaches the surface. Rhyolite is rarely produced at oceanic eruptions.
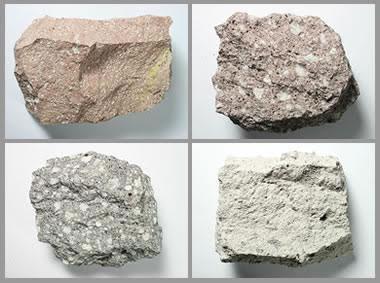
Figure: Rhyolite Porphyry: Several specimens of rhyolite porphyry, each about three inches across.
Eruptions of Granitic Magma
Eruptions of granitic magma can produce rhyolite, pumice, obsidian, or tuff. These rocks have similar compositions but different cooling conditions. Explosive eruptions produce tuff or pumice. Effusive eruptions produce rhyolite or obsidian if the lava cools rapidly. These different rock types can all be found in the products of a single eruption.
Eruptions of granitic magma are rare. Since 1900 only three are known to have occurred. These were at St. Andrew Strait Volcano in Papua New Guinea, Novarupta Volcano in Alaska, and Chaiten Volcano in Chile.
Granitic magmas are rich in silica and often contain up to several percent gases by weight. As these magmas cool, the silica starts to connect into complex molecules. This gives the magma a high viscosity and causes it to move very sluggishly.
The high gas content and high viscosity of these magmas are perfect for producing an explosive eruption. The viscosity can be so high that the gas can only escape by blasting the magma from the vent.
Granitic magmas have produced some of the most explosive volcanic eruptions in Earth's history. Examples include Yellowstone in Wyoming, Long Valley in California, and Valles in New Mexico. The sites of their eruption are often marked by large calderas.
Lava Domes
Sluggish rhyolitic lava can slowly exude from a volcano and pile up around the vent. This can produce a mound-shaped structure known as a "lava dome." Some lava domes have grown to a height of several hundred meters.
Lava domes can be dangerous. As additional magma extrudes, the brittle dome can become highly fractured and unstable. The ground can also change slope as the volcano inflates and contracts. This activity can trigger a dome collapse. A dome collapse can lower the pressure on the extruding magma. This sudden lowering of pressure can result in an explosion. It can also result in a debris avalanche of material falling from the tall collapsing dome. Many pyroclastic flows and volcanic debris avalanches have been triggered by a lava dome collapse.

Fire Opal is sometimes found filling cavities in rhyolite. This specimen of rhyolite has multiple vugs filled with gemmy transparent orange fire opal. This material can be cut into beautiful cabochons and is sometimes faceted when it is transparent or even translucent. Famous deposits of this type of fire-opal-in-rhyolite are found in Mexico. This photo is used here through a Creative Commons license. It was produced by Didier Descouens.
Rhyolite and Gemstones
Many gem deposits are hosted in rhyolite. These occur for a logical reason. The thick granitic lava that forms rhyolite often cools quickly while pockets of gas are still trapped inside of the lava. As the lava quickly cools, the trapped gas is unable to escape and forms cavities known as "vugs." Later, when the lava flow has cooled and hydrothermal gases or groundwater move through, the material can precipitate in the vugs. This is how some of the world's best deposits of red beryl, topaz, agate, jasper, and opal are formed. Gem hunters have learned this and are always on the lookout for vuggy rhyolite.

Rhyolite Arrowheads: Rhyolite was often used to make stone tools and weapons when more suitable materials were not available. It has been fashioned into scrapers, hoes, axe heads, spear points, and arrowheads.
Uses of Rhyolite
Rhyolite is a rock that is rarely used in construction or manufacturing. It is often vuggy or highly fractured. Its composition is variable. When better materials are not locally available, rhyolite is sometimes used to produce crushed stone. People have also used rhyolite to manufacture stone tools, particularly scrapers, blades, and projectile points. It was probably not their material of choice, but a material used out of necessity.
Q16) Explain Felsite and pegmite in detail.
A16) Felsite
Felsite is a term that has long been generally used by geologists, especially in England, to designate fine-grained igneous rocks of acid (or subacid) composition. As a rule, their ingredients are not determinable by the unaided eye, but they are principally felspar and quartz as very minute particles. The rocks are pale-colored (yellowish or reddish as a rule), hard, splintery, much jointed, and occasionally nodular. Many felsites contain porphyritic crystals of clear quartz in rounded blebs, more or less idiomorphic felspar, and occasionally biotite. Others are entirely fine-grained and microor cryptocrystalline. Occasionally they show a fluxional banding; they may also be spherulitic or vesicular. Those which carry porphyritic quartz are known as quartz-felsites; the term soda-felsites has been applied to similar fine-grained rocks rich in soda-felspar.
Although there are few objections to the employment of felsite as a field designation for rocks having the above characters, it lacks definiteness and has been discarded by many petrologists as unsuited for the exact description of rocks, especially when their microscopic characters are taken into consideration. The felsites accordingly are broken up into "graniteporphyries," "orthophyres" and "orthoclase-porphyries," "felsitic-rhyolites," "keratophyres," "granophyres," "microgranites". But felsite or microfelsite is still the generally accepted designation for that very fine-grained, almost cryptocrystalline substance that forms the ground-mass of so many rhyolites, dacites, and porphyries.
In the hand specimen, it is a dull, lustreless, stony-looking aggregate. Under the microscope even with high powers and the very thinnest modern sections, it often cannot be resolved into its components. In places it may contain determinable minute crystals of quartz; less commonly it may show grains which can be proved to be felspar, but usually, it consists of an ultra-microscopic aggregate of fibers, threads, and grains, which react to polarized light feebly and indefinitely. Spherulitic, spotted, streaky and fluidal structures may appear in it, and many different varieties have been established on such characters as these but without much validity.
Its association with the acid rocks, its hardness, method of weathering, and chemical composition, indicate that it is an intermixture of quartz and acid felspar, and the occasional presence of these two minerals in well-defined grains confirms this. Moreover, in many dikes, while the ground-mass is microcrystalline and consists of quartz and felspar near the center of the mass, towards the margins, where it has been rapidly chilled by contact with the cold surrounding rocks, it is felsitic. The very great viscosity of acid magmas prevents their molecules, especially when cooling takes place suddenly, from arranging themselves to form discrete crystals, and is the principal cause of the production of felsitic ground-masses. In extreme cases, these conditions hinder crystallization altogether, and glassy rocks result. Some rocks are felsitic in parts but elsewhere glassy, and it is not always clear whether the felsite is an original substance or has arisen by the devitrification of primary glass. The presence of perlitic structure in some of these felsites points to the latter conclusion, and the results of an examination of ancient glasses and of artificial glass which has been slowly cooled are under this view. It has been argued that felsite is a eutectic mixture of quartz and felspar, such that when solidification takes place and the excess of felspar (or quartz) has crystallized out it remains liquid till the temperature has fallen to its freezing point, and then consolidates simultaneously. This may be so, but analyses show that it has not always the same composition and consequently that the conditions which determine its formation are not quitesimple. Felsitic rocks are sometimes silicified and have their matrix replaced by granular aggregates of cloudy quartz.
Pegmatite
Pegmatites are extreme igneous rocks that form during the final stage of a magma’s crystallization. They are extreme because they contain exceptionally large crystals and they sometimes contain minerals that are rarely found in other types of rocks.
To be called a "pegmatite," rock should be composed almost entirely of crystals that are at least one centimeter in diameter. The name "pegmatite" has nothing to do with the mineral composition of the rock.

Figure: Topaz on albite
Most pegmatites have a composition that is similar to granite with abundant quartz, feldspar, and mica. These are sometimes called "granite pegmatites" to indicate their mineralogical composition. However, compositions such as "gabbro pegmatite," "syenite pegmatite," and any other plutonic rock name combined with "pegmatite" are possible.
Pegmatites are sometimes sources of valuable minerals such as spodumene (an ore of lithium) and beryl (an ore of beryllium) that are rarely found in economic amounts in other types of rocks. They also can be a source of gemstones. Some of the world’s best tourmaline, aquamarine, and topaz deposits have been found in pegmatites.
The Rock with Large Crystals
Large crystals in igneous rocks are usually attributed to a slow rate of crystallization. However, with pegmatites, large crystals are attributed to low-viscosity fluids that allow ions to be very mobile.
During the early stages of magma’s crystallization, the melt usually contains a significant amount of dissolved water and other volatiles such as chlorine, fluorine, and carbon dioxide. Water is not removed from the melt during the early crystallization process, so its concentration in the melt grows as crystallization progresses. Eventually, there is an overabundance of water, and pockets of water separate from the melt.
These pockets of superheated water are extremely rich in dissolved ions. The ions in the water are much more mobile than ions in the melt. This allows them to move about freely and form crystals rapidly. This is why crystals of a pegmatite grow so large.
The extreme conditions of crystallization sometimes produce crystals that are several meters in length and weigh over one ton. For example, a large crystal of spodumene at the Etta Mine in South Dakota was 42 feet long, 5 feet in diameter, and yielded 90 tons of spodumene!

Figure: Himalaya pegmatite