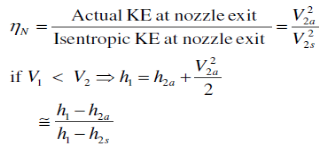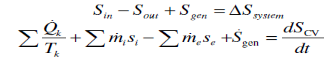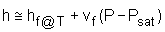When mass enters a system, the energy of the system increases because of the energy accompanied by mass. Also the energy of the system decreases when mass leaves the system Heat and work have been introduced in the previous sections. Systems that involve energy balance with mass flow are considered in the following section. Q3) Write Isentropic Efficiencies of Turbines? A3) The ratio of the actual work output of the turbine to the work output that would be achieved if the process between the inlet state and the exit pressure were isentropic
|
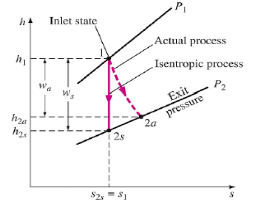
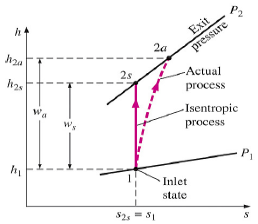
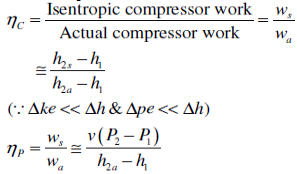
The ratio of the work input required to raise the pressure of a gas to a specified value in an isentropic manner to the actual work input
Fig 2
A realistic model process for compressors that are intentionally cooled during the compression process is the reversible isothermal process, defined as isothermal Efficiency
c = wt / wa
|
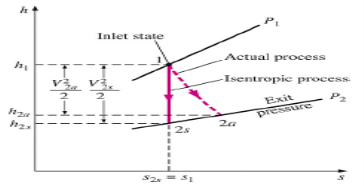
The ratio of the actual kinetic energy of the fluid at the nozzle exit to the kinetic energy value at the exit of an isentropic nozzle of the same inlet state and exit pressure
Fig 3
|
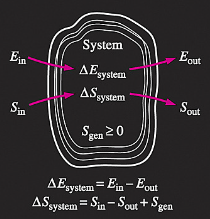
Increase of entropy principle for any system (Total entropy entering) – (total entropy leaving) + (total entropy generated) = (change in the total entropy of the system)
Fig 4
the entropy change of a system during a process is equal to the net entropy transfer through the system boundary and the entropy generated within the system Entropy change of the system
|
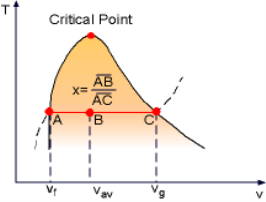
During the vaporization process of water, the substance is a mixture of saturated liquid and saturated vapor. Quality (x) is defined to describe the fraction of saturated vapor in the mixture. x = mvapor/mtotal Quality has a value between 0 and 1. x equals 1 for saturated vapor and x equals 0 for saturated liquid according to its definition. Saturated mixture is a two-phase system. For convenience, it can be treated as a homogenous mixture and the properties of this mixture are simply the average properties of the saturated liquid and saturated vapor. For example, the specific volume of the mixture can be determined by Vav = Vf + Vg mtotal vav = mf vf + mg vg Rearranging the above equation to give an expression for the quality x as x = (vav - vf)/vfg Based on this equation, quality can be determined from the horizontal distance on the T-v or P-v diagram as shown in the figure.
The above process can be repeated for u and h uav = uf + ufg x hav = hf + hfg x
|
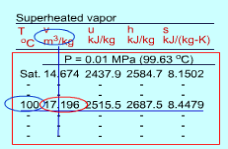
Since the superheated vapor is a single phase substance, the temperature and pressure are independent. In the superheated vapor tables, the properties are listed as a function of both the temperature and pressure. The tabulated properties include v, u, h and s. The saturation temperature corresponding to the pressure is given in the parentheses following the pressure value. Superheated vapor can be characterized by
h > hg at a given P or T
|
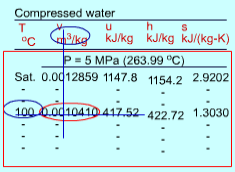
The format of the sub cooled liquid water tables is similar to the format of the superheated vapor tables. In the absence of compressed liquid data, a common practice is to use the saturated liquid data based on the given temperature to estimate the properties of compressed liquid. For h, the error can be reduced using the following approximation Sub cooled liquid can be characterized by
h < hf at a given P or T The entropy of a pure substance is determined from tables in the same manner as other properties such as v, u, and h. The value of entropy at a specified state is determined just like any other property.
If the compressed liquid data are not available, the entropy of the compressed liquid can be approximated by the saturated liquid at the same temperature
|