Module-5
Enzymes
Q1. Define catalysts?
A1. A catalyst is a substance that can be added to a reaction to increase the reaction rate without getting consumed in the process. Catalysts typically speed up a reaction by reducing the activation energy or changing the reaction mechanism. Enzymes are proteins that act as catalysts in biochemical reactions.
Common types of catalysts include enzymes, acid-base catalysts, and heterogeneous (or surface) catalysts.
Q2.How are Enzyme catalyzed reactions monitored?
A2. Monitoring the rate of an enzyme- that catalyses a reaction is called ‘enzyme kinetics’. The kinetics of an enzyme-catalysed reaction can indirectly provide information about the mechanism of catalysis. The rate or velocity of a reaction is the change in the concentration of reactant or product per unit of time
|
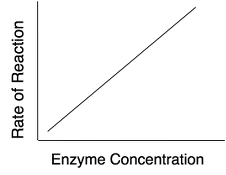
For a give enzyme concentration, the rate of reaction increases with increase in substrate concentration until all the available active sites are occupied by the substrates.
Once all the active sites are used up, the rate of reaction remains constant with increase in substrate concentration. Therefore, the theoretical maximum rate is never quite obtained. The extra substrate has to wait until the next enzyme/substrate complex release product before it takes part in another reaction.
|
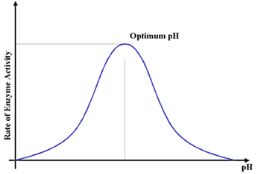
The rate of enzyme activity is maximum, at optimum ph.
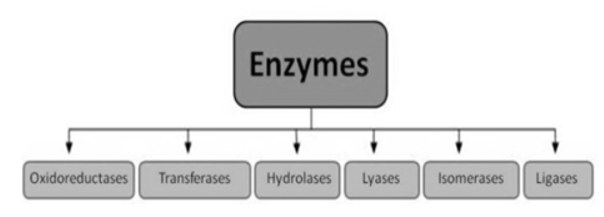
Q3. Illustrate Enzyme classification?
A3.
|
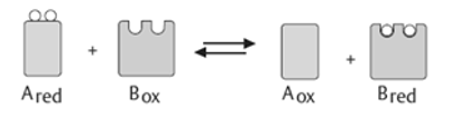
1).Oxidoreductases: These enzymes catalyse the transfer of oxygen or hydrogen atom or electrons from one substrate to the other. (Redox reactions).
E.g., Oxidases, Dehydrogenases, Oxygenises, Peroxidases, Catalases.
|
2). Transferases: The transfer of a functional group from one substrate to another is catalysed by this enzyme.E.g., Kinases, Transaminase.
|
3). Hydrolases: these enzymes catalyse the hydrolysis or breakdown of the substrate.
E.g., Lysozyme, digestive enzymes, acid phosphatase.
|
4). Isomerases: they catalyse intramolecular changes in the substrate.E.g., Isomerase, Fumarase.
|
5). Lyases: catalyses the non-hydrolytic removal of a group or addition of a group to a substrate.E.g., Decarboxylases, Aldolases.
|
6). Ligases (Synthetases): catalyses the joining of two molecules by forming new bonds.E.g., Citric acid synthetase.
|
thereby facilitating the flow of the reaction.
Types | Biochemical Property |
Oxidoreductases | The enzyme Oxidoreductase catalyses the oxidation reaction where the electrons tend to travel from one form of a molecule to the other. |
Transferases | The Transferases enzymes help in the transportation of the functional group among acceptors and donors’ molecules. |
Hydrolases | Hydrolases are hydrolytic enzymes, which catalyse the hydrolysis reaction by adding water to cleave the bond and hydrolyse it. |
Lyases | Adds water, carbon dioxide or ammonia across double bonds or eliminate these to create double bonds. |
Isomerases | The Isomerases enzymes catalyse the structural shifts present in a molecule, thus causing the change in the shape of the molecule. |
Ligases | The Ligases enzymes are known to charge the catalysis of a ligation process. |
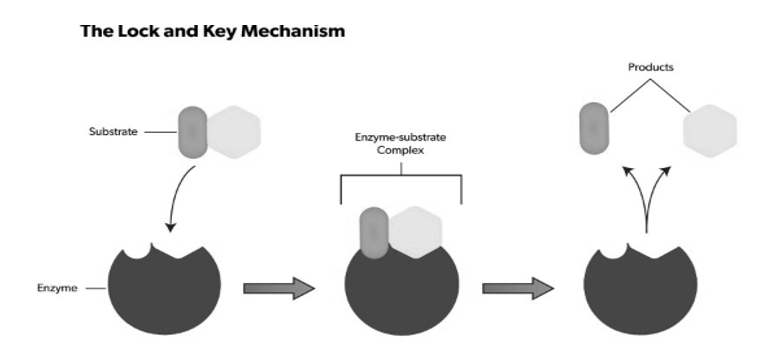
Q4. Explain the Lock and key mechanism of enzyme action?
A4. The lock-and-key analogy sees this process as very specific, further only a particular key can fit into the keyhole of the specific lock. If the key is in any way smaller, larger or simply a different shape, then it does not fit into the keyhole, and subsequently a reaction cannot take place. The theory was first described by Emile Fischer(lock-and-key analogy) in 1894, and since then many other theories to were discovered explain the mechanics of enzyme reactions.
|
The substrate binds to the active site, and a reaction takes place that ultimately causes the release of the formed product. Enzymes catalyse this reaction by facilitating chemical bond changes in the substrate through altering the distribution of electrons.
Q5. Write a short note on enzyme kinetics?
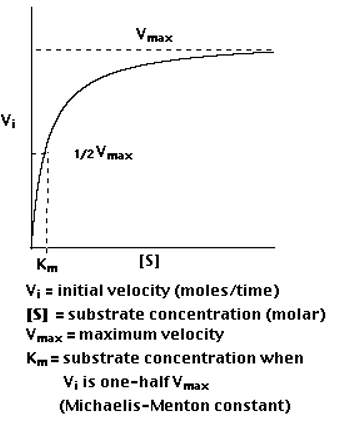
A5. The study of the rate at which an enzyme works is called enzyme kinetics. Enzyme kinetics as a function of the concentration of substrate available to the enzyme is observed here.
Plotting Vi as a function of [S], the following is observed
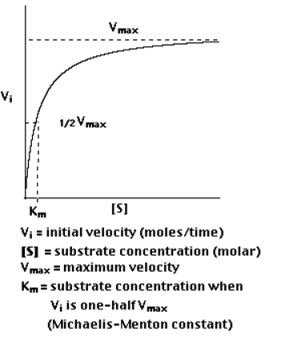
Km is (roughly) an inverse measure of the affinity or strength of binding between the enzyme and its substrate. The lower the Km, the greater the affinity (so the lower the concentration of substrate needed to achieve a given rate of reaction).
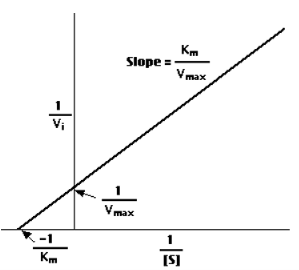 a "double-reciprocal" or Lineweaver-Burk plot is obtained byplotting the reciprocals of the same data points. This provides a more precise way to determine Vmax and Km.
a "double-reciprocal" or Lineweaver-Burk plot is obtained byplotting the reciprocals of the same data points. This provides a more precise way to determine Vmax and Km.
The Effects of Enzyme Inhibitors
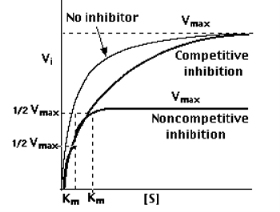 Enzymes can be inhibited
Enzymes can be inhibited
The distinction can be determined by plotting enzyme activity with and without the inhibitor present.
In the presence of a competitive inhibitor, it takes a higher substrate concentration to achieve the same velocities that were reached in its absence. So,while Vmax can still be reached if sufficient substrate is available, one-half Vmax requires a higher [S] than before and thus Km is larger.
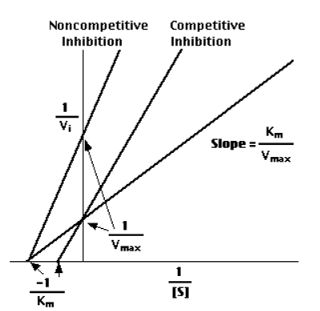 With non-competitive inhibition, enzyme molecules that have been bound by the inhibitor are not taken into consideration.
With non-competitive inhibition, enzyme molecules that have been bound by the inhibitor are not taken into consideration.
Enzymes are protein catalysts that, like all catalysts, speed up the rate of a chemical reaction without being themselves used up in the process.
They achieve their effect by temporarily binding to the substrate and, thereby, lower the activation energy needed to convert it to a product.
Q6. Factors that influence Enzyme activity?
A6. The rate at which an enzyme works is influenced by many important factors, e.g.,
(when their availability is more, the quicker the enzyme molecules collide and bind with them). The concentration of substrate is designated [S] and is expressed in units of molarity.
As the temperature rises, molecular motion also increases — and therefore collisions between enzyme and substrate — speed up. But as enzymes are proteins, there is an upper limit beyond which the enzyme becomes denatured and ineffective high temperatures can denature proteins.
- competitive inhibitors are molecules that bind to the same site as the substrate — preventing the substrate from binding to the enzyme active site — but are not changed by the enzyme.
- non-competitive inhibitors are molecules that bind to some other site on the enzyme reducing the power of the catalysis.
pH influences the conformation of a protein and as enzyme activity is crucially dependent on protein conformation, its activity is affected accordingly.
Q7. What is An Induced fit theory?
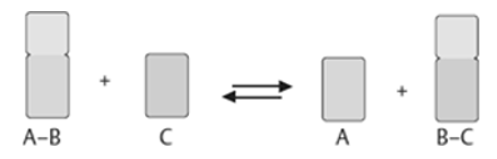
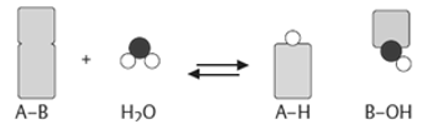
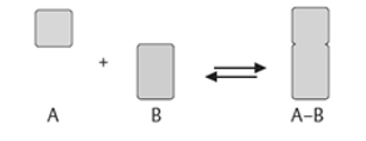
A7. An enzyme attracts substrates to its active site, and catalyses the chemical reaction by during which products are formed, and then the products that are formed dissociate (separate from the enzyme surface). The combination formed by the substrate and its enzyme called the enzyme–substrate complex. When two substrates and one enzyme are involved in a reaction, the complex is called a ternary complex; A binary complex is formed when one substrate and one enzyme are involved in a reaction. The substrates are attracted to the active site by hydrophobic and electrostaticand forces, which are called noncovalent bonds because they are physical attractions and not chemical bonds.
|
In the induced-fit theory of enzyme-substrate binding, a substrate approaches the surface of an enzyme (step 1 in box A, B, C) and causes a change in the enzyme shape that results in the correct alignment of the catalytic groups (triangles A and B; circles C and D represent substrate-binding groups on the enzyme that are essential for catalytic activity). The catalytic groups react with the substrate to form products (step 2). The products then separate from the enzyme, freeing it to repeat the sequence (step 3). Boxes D and E represent examples of molecules that are too large or too small for proper catalytic alignment. Boxes F and G demonstrate binding of an inhibitor molecule (I and I′) to an allosteric site, thereby preventing interaction of the enzyme with the substrate. Box H illustrates binding of an allosteric activator (X), a non-substrate molecule capable of reacting with the enzyme.
Q8.Explain the difference between Catalyst and an Enzyme?
A8. Catalyst and enzyme are two substances that increase the rate of a reaction without being changed by the reaction. There are two types of catalysts as enzymes and inorganic catalysts. Enzymes are a type of biological catalysts. The main difference between catalyst and enzyme is that catalyst is a substance that increases the rate of a chemical reaction whereas enzyme is a globular protein that can increase the rate of biochemical reactions. The inorganic catalysts include mineral ions or small molecules. In contrast, enzymes are complex macromolecules with 3D structures. Enzymes are specific and work in mild conditions.
Catalyst | Enzyme |
Catalyst defined as the molecules that speed up the rate of a reaction without having a change in its structure. | An enzyme is known as a Biological catalyst and globular protein that speed up natural reactions. |
Correlation | |
Could either be enzymes or inorganic salts | Considered as a type of a Catalyst |
Type | |
Mineral ions or small molecules | Globular proteins |
Size Difference | |
Similar in size to the molecule of substrate | Very larger as compared to the substrate molecule |
Molecular Weight | |
The molecular weight is low | The molecular weight of enzymes are high |
Action | |
Normally act on physical reactions | Always act on biochemical Reactions |
Efficiency | |
Work less efficiently | Work highly efficiently |
Specificity | |
Can maximize the rate of various set of reactions | Can only act and increase the rate of a particular reaction |
Regulator Molecules | |
Cannot control the function of inorganic catalysts | Can regregulate the function of enzymes by binding bindingof regulatory molecules with the specific enzyme |
Temperature | |
Not sensitive to small temperature changes, so they work at high temperatures | Temperature specific, so at low temperature, enzymes become inactive, and at high temperature, enzymes get denatured |
pH | |
Not usually sensitive to small changes occurring in pH | Sensitive to small pH changes and operate only at a specific range of pH |
Pressure | |
Work only at high pressure | Work only at normal pressure |
Protein Poisons | |
Protein poisons contain no effect | Can be affected and poisoned by protein poisons |
Short Wave Radiations | |
Contain no effect on the inorganic catalysts | Can have denatured the enzymes |
Examples | |
Iron, platinum, and vanadium oxide | Glucose-6-phosphate, alcohol dehydrogenase, amylase, lipase, and aminotransferase |
Q9. Define an Active site?
A9. To catalyse a reaction, an enzyme will grab on (bind) to one or more reactant molecules. These molecules are the enzyme's substrates.
In some reactions, one substrate is broken down into multiple products. In others, two substrates come together to create one larger molecule or to swap pieces. In fact, whatever type of biological reaction you can think of, there is probably an enzyme to speed it up!
The part of the enzyme where the substrate binds is called the active site (since that’s where the catalytic “action” happens).
The set of amino acids found in the active site, along with their positions in 3D space, give the active site a very specific size, shape, and chemical behaviour, these amino acids, an enzyme's active site is uniquely suited to bind to a particular target—the enzyme's substrate or substrates—and help them undergo a chemical reaction.